��Ŀ����
����Ŀ�������������壨FeC2O42H2O����һ��dz��ɫ���壬������ˮ�������ֽ⣬������﮵�ص�ԭ���ϣ�ij��ѧ��ȤС�����ʵ����֤�������������ȷֽ���������������ط���TG��ȷ����ֽ�����ù���������ɣ�̽���������£�
���������ϣ�
������ͭ���壨CuSO45H2O������ʱ�ᷢ�����·�Ӧ�� CuSO45H2O![]() CuSO4+5H2O�����������壨FeC2O42H2O��Ҳ�ܷ������Ƶķ�Ӧ��
CuSO4+5H2O�����������壨FeC2O42H2O��Ҳ�ܷ������Ƶķ�Ӧ��
�ڲ������������ȷֽ�����������H2O��CO��CO2��
�۰�ɫ����ˮCuSO4��ˮ����������ɫ��
��Ũ���������ˮ�ԣ��dz��õ�����������
��Ũ������������Һ�����մ����Ķ�����̼���壬��ʯ��ˮ����Ч���á�
��ʵ����ƣ�
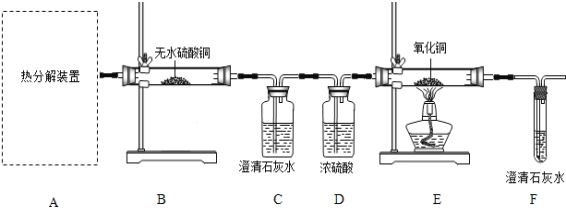
��1���ӻ����Ƕȿ��ǣ�����ʵ��װ�õ�����ȱ����_______��
��2����ѡ������ʵ��װ���е�_____��ѡ��ס����ҡ�����Ϊ�����еġ��ȷֽ�װ�á���
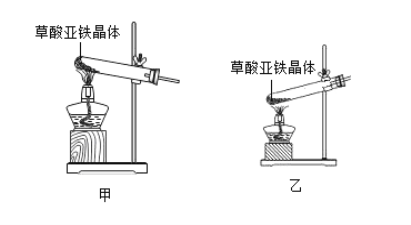
���������ۣ�
��3��ʵ�����������۲쵽��������B�а�ɫ��ˮCuSO4�����ɫ��___________��E�к�ɫ��ĩ��ɺ�ɫ�����֤���������������ȷֽ�����������H2O��CO��CO2��д��Fװ������������Ӧ�Ļ�ѧ����ʽ_________��
��4������ȤС���������ʵ��ʱ��Ӧ�ȵ�ȼ__________�����A����E�����ľƾ��ƣ�ԭ����____________��
��5����ͨ��Fװ���г���ʯ��ˮ�������֤���������������ȷֽ�������������CO�������ʵ����Ʒ������иĽ�����˵����ĸĽ���ʩ________��
�����ݷ�����
��6����ȡ3.60g�����������壨FeC2O42H2O����Է���������180�������ط���������ȷֽ⣬�õ�ʣ�������������¶ȱ仯��������ͼ��ʾ���������ͼ�ش��������⣺
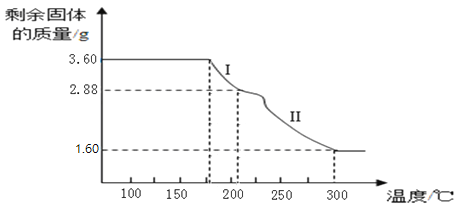
�ٹ��̢�����Ӧ�Ļ�ѧ����ʽΪ____________��
����֪��300��ʱ��������������ȫ�ֽ��ʣ�����ֻ��һ���������������ͨ�������ȷ����������Ϊ_____________�������ƣ�������д��������̣�
���𰸡���β������װ�� �� C�г���ʯ��ˮ����� CO+ CuO ![]() Cu + CO2 A ���Ƚ�װ���ڵĿ����ž�����ֹCO�������ϼ��ȷ�����ը ��C��Dװ��֮������ʢ������Ũ����������Һ��ϴ��ƿ FeC2O42H2O
Cu + CO2 A ���Ƚ�װ���ڵĿ����ž�����ֹCO�������ϼ��ȷ�����ը ��C��Dװ��֮������ʢ������Ũ����������Һ��ϴ��ƿ FeC2O42H2O![]() FeC2O4+2H2O ������(�������)
FeC2O4+2H2O ������(�������)
��������
��1�����ݲ������Ͽ�֪���������������ȷֽ�����������H2O��CO��CO2��һ����̼�ж�����ɢ�������л���Ⱦ�������ӻ����Ƕȿ��ǣ�����ʵ��װ�õ�����ȱ��������β��װ�ã�
��2�����ȹ�������ʱ��Ϊ��ֹ����ˮ���������Թܵ�ը�ѣ��Թܿ�Ӧ����������б�����ȷֽ�װ�á�Ӧ��ѡ���װ�ã�
��3������ˮ���������ݲ������Ͽ�֪����ɫ����ˮCuSO4��ˮ����������ɫ��������ˮCuSO4�����Ƿ���ˮ���ɣ����ж�����̼������������̼��ʹ����ʯ��ˮ�ͻ��ǣ����ó���ʯ��ˮ���������̼�Ĵ��ڣ�����CO������CO��������ͭ��Ӧ����ͭ�Ͷ�����̼���ɸ�������ͭ�Ƿ�ԭ����һ����̼�Ĵ��ڣ����Ը���ʵ�����������۲쵽��������B�а�ɫ��ˮCuSO4�����ɫ��C�г���ʯ��ˮ�������E�к�ɫ��ĩ��ɺ�ɫ�����֤���������������ȷֽ�����������H2O��CO��CO2��Fװ������������Ӧ��һ����̼������ͭ�ڼ��ȵ�����������ͭ�Ͷ�����̼����ѧ����ʽCO+ CuO ![]() Cu + CO2��
Cu + CO2��
��4����������ʵ��ʱ��Ӧ�ȵ�ȼA���ľƾ��ƣ�ԭ�������Ƚ�װ���ڵĿ����ž�����ֹCO�������ϼ��ȷ�����ը��
��5����ͨ��Fװ���г���ʯ��ˮ�������֤���������������ȷֽ�������������CO������C��Dװ��֮������ʢ������Ũ����������Һ��ϴ��ƿ���Գ�ȥ������̼���壻
��6����3.60g�������������в�����������Ϊ=![]() ���ᾧˮ����=3.60g-2.88g=0.72g���������̢�ʣ����������Ϊ2.88g��˵���ᾧˮ��ȫʧȥ����ʱ�Ĺ�������ΪFeC2O4�����̢�ķ�Ӧ�Dz��������������ȷֽ����ɲ���������ˮ����Ӧ�Ļ�ѧ����ʽ��FeC2O42H2O
���ᾧˮ����=3.60g-2.88g=0.72g���������̢�ʣ����������Ϊ2.88g��˵���ᾧˮ��ȫʧȥ����ʱ�Ĺ�������ΪFeC2O4�����̢�ķ�Ӧ�Dz��������������ȷֽ����ɲ���������ˮ����Ӧ�Ļ�ѧ����ʽ��FeC2O42H2O![]() FeC2O4+2H2O��
FeC2O4+2H2O��
��3.60g����������������Ԫ������=![]() ������������������Ԫ������=1.60g-1.12g=0.48g����ԭ�Ӻ���ԭ�ӵĸ�����=
������������������Ԫ������=1.60g-1.12g=0.48g����ԭ�Ӻ���ԭ�ӵĸ�����=![]() ��������������ﻯѧʽ��Fe2O3�����ʵ���������������
��������������ﻯѧʽ��Fe2O3�����ʵ���������������