��Ŀ����
����Ŀ��ѧϰ��ȤС��Ա�����̼��Ƶĺ�������̽����
��������⣩���ѡ��ҩƷ�����װ�ý��вⶨ��
���������ϣ����ǵ���Ҫ�ɷ���CaCO3�������ɷֶ�ʵ��Ӱ����Բ�������ʱ��CaCO3������ˮ��CaSO4����ˮ
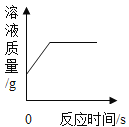
�������ʵ�飩ʵ��һ��ѡ��ҩƷ���ֱ�ȡ����������״�ͷ�ĩ״�ı�����Ʒ����������Ũ�ȵ�ϡ������ͼ1��������ƿ�з�Ӧ���ɼ�����

��1��ͼ1�з�Ӧ�Ļ�ѧ����ʽ��_________
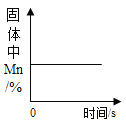
��2����ͼ2������ѡ���ĩ״��Ʒ��������________
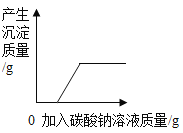
��3����ͼ3������Ӱ��ʵ��ⶨȷ�Ե�ԭ��һ��ˮ�����������ӣ�����_____
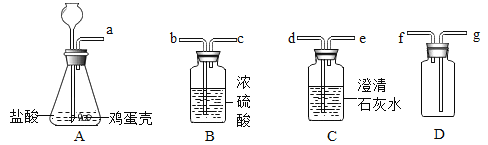
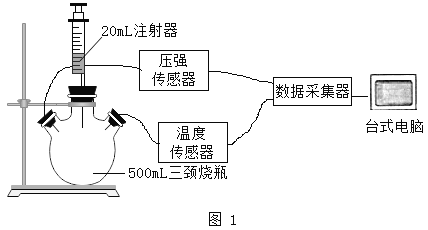
ʵ��������װ�á�С�������ͼ4װ�ý��вⶨ
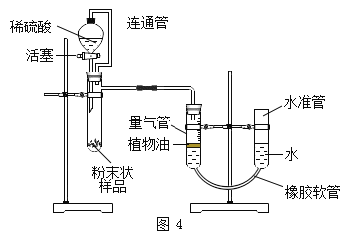
��4��������ϡ��������Թܣ��۲쵽��������_________�� ��Ӧ��������ж���������ǰ����ˮ����������Һ����ƽ��ԭ����_____
ʵ�������Ż�װ�á�����ʦָ���£�С���Ż��������ͼ5װ�ý��вⶨ
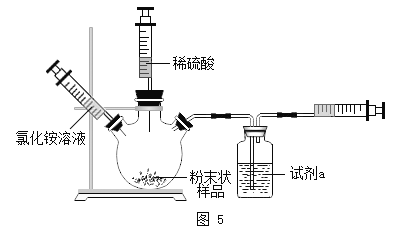
��5���Լ�a��__________
��6������װ�������Եķ�����________
��7����������ϡ���ᣬһ��ʱ���Ӧֹͣ���ټ����Ȼ����Һ�������ֲ������ݣ��Ʋ�����Ȼ�淋�������__________
�����ݴ�����
��8��ʵ�����У���Ʒ����Ϊm g������ϡ�������ΪV1mL�������Ȼ����Һ���ΪV2 mL���Ҳ���Ͳ������ΪV mL����Ӧ����CO2���Ϊ______����ʵ��������CO2�ܶ�Ϊd g/mL������Ʒ̼���������������ʽΪ________
����˼�����ۣ�
��9����ʵ�����ȣ�ʵ�������ŵ���______��дһ�㣩��
���𰸡�CaCO3+2HCl=CaCl2+H2O+CO2�� ��λʱ���ڣ���ӦЧ�ʸ��� Ũ����ӷ�������HCl�������� �Թ��г������ݣ���������Һ���½���ˮ����Һ������ ʹ����ѹǿƽ�� Ũ���� ���Ҳ�ע�����Ļ��������������֣������ָ���ԭ�� �ٽ��ܵ�������ܽ⣬ʹ���������̼��Ʒ�Ӧ VmL��V1mL+V2mL�� ![]() ��100% ��Ʒ�е�̼�����ȫ����ϡ���ᷴӦ���ⶨ�Ľ����ȷ
��100% ��Ʒ�е�̼�����ȫ����ϡ���ᷴӦ���ⶨ�Ľ����ȷ
��������
��1���������֪��ͼ1�еķ�Ӧ�DZ����е�̼��������ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼���ʷ�Ӧ�Ļ�ѧ����ʽдΪ��CaCO3+2HCl=CaCl2+H2O+CO2����
��2����ͼ2��֪����ĩ״���������ᷴӦ��ʱ��̣�˵����λʱ���ڣ���ӦЧ�ʸ��ߣ����λʱ���ڣ���ӦЧ�ʸ��ߡ�
��3����ͼ3��֪���淴Ӧʱ����ӳ���Ũ����ӷ�������HCl�������ӣ�Ҳ��Ӱ��ʵ��ⶨȷ�ԣ�����Ũ����ӷ�������HCl�������ӡ�
��4��ϡ������̼��Ʒ�Ӧ��������ơ�ˮ�Ͷ�����̼���۲쵽���������Թ��г������ݣ���������Һ���½���ˮ����Һ�������������Թ��г������ݣ���������Һ���½���ˮ����Һ��������
�������֪��Ӱ��ʵ��ⶨȷ�Ե����ذ���ѹǿ������ǰ����ˮ����������Һ����ƽ����ʹ����ѹǿƽ�⣬����ѹǿ��ͬ��ʵ���ȷ�Բ������ţ�����ʹ����ѹǿƽ�⡣
��5���Լ�aΪŨ���ᣬ��Ũ�����������ɶ�����̼�е�ˮ�֣�����ˮ�ֶԲⶨ������̼�������ţ�����Ũ���ᡣ
��6������װ�������Եķ���������װ�ã����Ҳ�ע�����Ļ��������������֣���������ָ���ԭ����֤��װ�ò�©��������Ҳ�ע�����Ļ��������������֣������ָ���ԭ����
��7���Ȼ����Һ�������ֲ������ݣ�˵�������Ȼ�狀�������̼��Ƽ�����Ӧ��˵���Ȼ���ܹ��ٽ�����Ƶ��ܽ⣬����ٽ��ܵ�������ܽ⣬ʹ���������̼��Ʒ�Ӧ��
��8��ʵ����ռ������������������̼�Ϳ��������п��������=V1mL+V2mL�����Է�Ӧ����CO2���=VmL��V1mL+V2mL��������VmL��V1mL+V2mL����
�ɻ�ѧ����ʽCaCO3+H2SO4=CaSO4+H2O+CO2����֪���μӷ�Ӧ��̼��������ɵĶ�����̼��������=100��44������̼��Ƶ�����Ϊ![]() g����Ʒ̼���������������ʽ=
g����Ʒ̼���������������ʽ=![]() ��100%������
��100%������![]() ��100%��
��100%��
��9����ʵ�����ȣ�ʵ�������ŵ�����Ʒ�е�̼�����ȫ����ϡ���ᷴӦ�������Ľ����ȷ��������Ʒ�е�̼�����ȫ����ϡ���ᷴӦ���ⶨ�Ľ����ȷ��

����Ŀ��ij�����ڵ�ȼ�������·�����Ӧ��������ͷ�Ӧ�ﹲ���֣����ǵ���ʾ��ͼ�ͷ�Ӧǰ������������ʾ��
������� | �� | �� | �� | �� |
|
��ʾ��ͼ |
|
|
|
| |
��Ӧǰ����/g | 68 | 100 | 1 | 0 | |
��Ӧ������/g | 0 | x | y | z |
��1����![]() ���ɵ�������
���ɵ�������![]() ��������Ԫ�صĻ��ϼ�Ϊ_____��
��������Ԫ�صĻ��ϼ�Ϊ_____��
��2�����е����������У��������������_____���ѧʽ����
��3��������Ӧ�Ļ�ѧ����ʽΪ_______________________��
����Ŀ������ƿ��������������Һ���ܱ��ʡ��±��з�������ƴ������
ѡ�� | ���� | ��������� |
A | ��� | 2NaOH + CO2 = Na2CO3 + H2O |
B | �Ƿ���� | ȡ������������ϡ���ᣬ�۲��Ƿ�������� |
C | �Ƿ�ȫ������ | ȡ�������������Ȼ�����Һ���۲������������ |
D | ��γ�ȥ���� | ȡ����������������������Һ������ |
A.AB.BC.CD.D
����Ŀ���������ƣ�![]() ����һ�ֵ���ɫ���壬��������������еĹ�������ij��ѧ�о�С��Թ������Ƶ��Ʊ���ʵ�����й�������ҩƷ�ijɷֽ���������̽����
����һ�ֵ���ɫ���壬��������������еĹ�������ij��ѧ�о�С��Թ������Ƶ��Ʊ���ʵ�����й�������ҩƷ�ijɷֽ���������̽����
���������ϣ����ڵ�ȼ�����£� ���ڿ�����ȼ�����ɴ��Ƚϸߵ�![]()
�ڹ��������ܺ�ˮ�Լ�������̼�������»�ѧ��Ӧ��
![]() ��
��
![]() .
.
�۽�������ˮ�ᷢ�����ҷ�Ӧ����![]()
��.�������Ƶ��Ʊ���
�о�С�������ͼװ���Ʊ��������ơ�
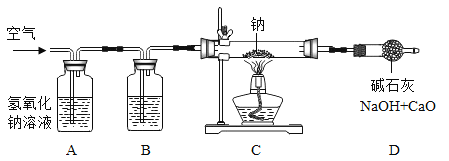
��1��װ��B�е��Լ���________��
��2��װ��C�з�Ӧ�Ļ�ѧ����ʽΪ________��
��3��װ��D��������________��
��.�ⶨ���õĹ���������Ʒ�ijɷ֣�
�����Լ�⣩�����Ʒ�ijɷ�
��4���벹���������ʵ�鱨���е�������ݡ�
ʵ����� | ʵ�鲽�� | ʵ������ | ���ͻ���� |
�� | ȡ����������Ʒ���Թ��У�����������ˮ | ��Ʒȫ���ܽ⣬�����ݲ��� | ��Ʒ�в���__�� |
�� | ȡ�������е���Һ���Թ��У���������� | �а�ɫ�������� | ��ѧ����ʽΪ_�� |
�� | ���ڳ�־��ú����ϲ���Һ�е���________�� | _____�� | ������������ |
��������⣩���ҩƷ����ɡ�
��5��ʵ��ܣ���ȡ10g��������������Ʒ���Թ��У�����������ϡ���ᣬ������������ȫ�����뵽�����ij���ʯ��ˮ�У����ˡ�ϴ�ӡ�����õ�̼��ƹ���5.00g������ȷ����Ʒ���������Ƶ���������_______��д��������̣�
����Ŀ��ij�ܱ���������X��O2��NO�������ʣ���һ�������³�ַ�Ӧ����÷�Ӧǰ������ʵ��������±������ݱ�����Ϣ���ж�����˵����ȷ����
���� | X | O2 | NO | H2O |
��Ӧǰ����/g | 17 | 70 | 1 | 0 |
��Ӧ������/g | 0 | ���� | 31 | 27 |
A.�÷�ӦΪ�û���Ӧ
B.X�Ļ�ѧʽ������NH3
C.������������ֵΪ36
D.��Ӧ���ɵ�NO��H2O�ĸ�����Ϊ3��2