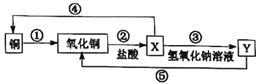
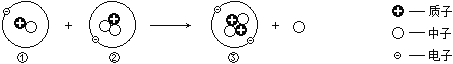
题目内容
【题目】为测定铜锌合金中锌的质量分数,进行了以下实验;取40g铜锌合金放入烧杯中,称得烧杯及所盛铜锌合金的总质量为140g,再把120g盐酸平均分成四份依次加入烧杯中,每次充分反应后进行称量,实验数据如下:
所加盐酸的次数 | 第一次 | 第二次 | 第三次 | 第四次 |
烧杯及所盛物质的总质量/g | 169.7 | 199.4 | 229.2 | 259.2 |
请你据此分析计算:
(1)第一次实验生成氢气的质量是 ________ g.
(2)利用第一次实验的数据,计算30g盐酸中溶质的质量分数________ (写出计算过程,精确到0.1%)
(3)实验最终结束后,同学们求出了合金中锌的质量分数=__________.
(4)下图表示所加盐酸的质量与反应后所得溶液质量的关系曲线,请写出A点(X=______ ,Y=______)的坐标。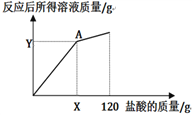
【答案】 0.3 36.5% 65% 80 105.2
【解析】本题考查了表格型计算题,质量分数和化学方程式的综合计算,处理数据时,当反应前后出现了质量差,要想到产生的气体。
(1)第一次实验生成氢气的质量=140g+30g-169.7g=0.3g;
(2)设:30克盐酸中所含HCl的质量为x。
Zn+2HCl=ZnCl2+H2↑
73 2
x 0.3g
![]() x=10.95g
x=10.95g
盐酸中溶质的质量分数=![]() ×100%=36.5%;
×100%=36.5%;
(3)实验最终结束后,产生的氢气质量=140g+120g-259.2g=0.8g;
设:生成0.8g的氢气需要锌的质量为y,HCl的质量为z。
Zn+2HCl=ZnCl2+H2↑
65 73 2
y z 0.8g
![]() y=26g
y=26g
合金中锌的质量分数=![]() ×100%=65%;
×100%=65%;
(4)根据关系曲线可知A点表示盐酸与锌恰好完全反应。
![]() z=29.2g,盐酸的质量=
z=29.2g,盐酸的质量=![]() =80g;反应后的溶液质量=26g+80g-0.8g=105.2g。所以A点坐标是(80,105.2)。
=80g;反应后的溶液质量=26g+80g-0.8g=105.2g。所以A点坐标是(80,105.2)。

【题目】在一次用餐中,同学们对燃料“固体酒精”产生了好奇,于是对其成分进行研究。
【查阅资料】①该固体酒精是用酒精、氯化钙和氢氧化钠按一定的质量比混合制成。
②氯化钙、氯化钡溶液均呈中性。
【提出问题】①酒精中是否含有碳元素?
②固体酒精中的氢氧化钠是否变质?
【实验探究】(1)按下图实验,发现澄清石灰水变浑浊,可得出酒精中含有碳元素的结论.此结论_____________(选填“合理”或“不合理”).
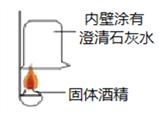
(2)取少量固体酒精于烧杯中,加足量的水充分溶解后静置,发现烧杯底部有白色沉淀.取沉淀于试管中加稀盐酸,有气泡产生.写出产生沉淀的化学方程式______________________。分析实验并结合资料得出氢氧化钠已变质。
(3)为进一步确定氢氧化钠是否完全变质,进行了如下探究.请完成实验报告
实验步骤 | 实验现象 | 实验结论 |
取步骤(2)烧杯中的上层清液于试管中。 ①滴入__________,振荡,静置。 | ______________________。 | 说明清液中还有碳酸钠 说明清液中还含有氢氧化钠,固体酒精中的氢氧化钠是部分变质 |
②______,观察现象。 | _________________ |