��Ŀ����
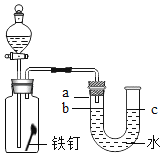
����Ŀ��С���ڿ������ϵ�֪������ͭ���ȿ��Էֽ⡣Ϊ��̽��������ͭ�ķֽ⼰����С���Ϳ���С���ͬѧ���������е�ʵ��̽��������һ����벢��ɶ�Ӧ���⣺

��1��ʵ�����е�һ�����Դ�����_____��
��2��С��˵ʵ����õ��ĺ�ɫ�������������ͭҲ���ܺ���̼�ۣ�С��˵�����ܺ���̼�ۣ�С����������_____��
��3��Ϊ�˽�һ��̽����ɫ�������Ҫ�ɷ֣�С���ö�װ�ü���ʵ�飺������ɫ�����ľ̿�ۻ�Ͼ���װ���Թ��м��ȣ�һ��ʱ���۲쵽�Թ���_____������ʯ��ˮ����ǣ�������˵����ɫ����������ͭ��д����ɫ���巢����Ӧ�Ļ�ѧ����ʽ��_____��
��4��С��˵Ҳ����������һ�ֲ�����ȵķ�����֤����ɫ����������ͭ��С������Ҫʵ�鲽��������ǣ�_____��
��5��ͨ����������С����֪������������ͭ��������ͭ��ͬʱ������ˮ������д���÷�Ӧ�Ļ�ѧ����ʽ_____�����С���ó����ۣ����ڼ��ȵ�����¶���ֽ����ɶ�Ӧ�Ľ����������ˮ�������ж�С���Ľ�����ȷ���˵�����ɣ�_____��
���𰸡�©���¶�û�н����ձ��ڱ� ��Ӧ���в�����̼Ԫ�� ��ɫ������ɫ 2CuO+C![]() 2Cu+CO2�� ȡ������ɫ�������Թ��У���������ϡ���ᣬ��ɫ�����ܽ⣬��Һ����ɫ����ɫ Cu��OH��2
2Cu+CO2�� ȡ������ɫ�������Թ��У���������ϡ���ᣬ��ɫ�����ܽ⣬��Һ����ɫ����ɫ Cu��OH��2![]() CuO+H2O ����ȷ��������Ϊ�����Եļ����ȷֽ����ɶ�Ӧ�������������ˮ�ļ����Ȳ��ֽܷ�
CuO+H2O ����ȷ��������Ϊ�����Եļ����ȷֽ����ɶ�Ӧ�������������ˮ�ļ����Ȳ��ֽܷ�
��������
��1��ʵ�����е�һ�����Դ�����©���¶�û�н����ձ��ڱڣ�
��2��С��˵ʵ����õ��ĺ�ɫ�������������ͭҲ���ܺ���̼�ۣ�С��˵�����ܺ���̼�ۣ�С���������Ƿ�Ӧ���в�����̼Ԫ�أ���Ӧ��������̼��
��3������ɫ�����ľ̿�ۻ�Ͼ���װ���Թ��м��ȣ�һ��ʱ���۲쵽�Թ��к�ɫ������ɫ������ʯ��ˮ����ǣ�˵����Ӧ����ͭ�Ͷ�����̼��������˵����ɫ����������ͭ��������Ӧ�Ļ�ѧ����ʽ��2CuO+C![]() 2Cu+CO2����
2Cu+CO2����
��4��С������Ҫʵ�鲽��������ǣ�ȡ������ɫ�������Թ��У���������ϡ���ᣬ��ɫ�����ܽ⣬��Һ����ɫ����ɫ������Ϊ����ͭ��ϡ���ᷴӦ��������ͭ��ˮ��
��5������������ͭ��������ͭ��ͬʱ������ˮ���÷�Ӧ�Ļ�ѧ����ʽ��Cu��OH��2![]() CuO+H2O��С���Ľ��۲���ȷ�����ɣ������Եļ����ȷֽ����ɶ�Ӧ�������������ˮ�ļ����Ȳ��ֽܷ⡣
CuO+H2O��С���Ľ��۲���ȷ�����ɣ������Եļ����ȷֽ����ɶ�Ӧ�������������ˮ�ļ����Ȳ��ֽܷ⡣

 ѧ���쳵�����ּ��ں�����ҵϵ�д�
ѧ���쳵�����ּ��ں�����ҵϵ�д�����Ŀ������ʵ���У���Ӧ������ͽ��۶���ȷ�����߾��������ϵ���ǣ� ��
ʵ����� | ���� | ���� | |
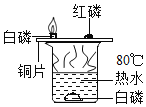
A | �����£������������� | ����ȼ�գ���������ɫ���� | ������������Ӧ |
B | ��ij��ɫ��Һ�е��� | �а�ɫ�������� | ����Һ��һ������̼���� |
C | �� | �����ݲ��� | �����ӷ� |
D | ���Ȼ�����Һ���е��������飬�۲�С�����Ƿ���� | С���ݱ��� | �Ȼ�����Һ�д��������ƶ������� |
A.AB.BC.CD.D
����Ŀ����1������������ȼ�յĻ�ѧ����ʽ�ǣ�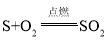 ����ʾ�˲��뷴Ӧ�ĸ�����֮���������ϵ�����ڵ�ȼ�����£�ÿ____������������____������������ǡ����ȫ��Ӧ����_____�������Ķ�������
����ʾ�˲��뷴Ӧ�ĸ�����֮���������ϵ�����ڵ�ȼ�����£�ÿ____������������____������������ǡ����ȫ��Ӧ����_____�������Ķ�������
��2����ѧ��Ӧǰ��ض�û�з����ı����______������ĸ��
��ԭ����Ŀ �ڷ�����Ŀ ��Ԫ������ ���������� ��ԭ������ �����ʵ�������
A �٢ܢ�
B �٢ۢݢ�
C �٢ڢ�
D �ڢۢ�
��3��ij������X�ڿ�������ȫȼ�գ���Ӧ�Ļ�ѧ����ʽΪ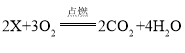 ����X�Ļ�ѧʽΪ____________��
����X�Ļ�ѧʽΪ____________��
��4���ܱ��������мס����������ʸ�10g������һ��ʱ����������и����ʵ��������±���ʾ��
���� | �� | �� | �� | �� |
��Ӧ�������/g | 1.5 | X | 0.8 | 2.2 |
����ѡ����ȷ������________��
A �÷�ӦΪ���Ϸ�Ӧ
B X=54
C ������һ���ǵ���
D ��һ���ǻ�����
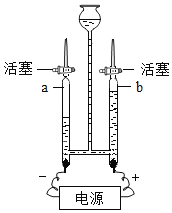
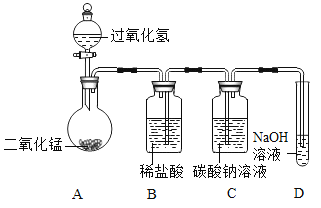
����Ŀ��ѧϰ�˶������̶Թ�������ֽ��д����õ�֪ʶ��ijͬѧ�룺����ͭ�ܷ������ƶ������̵Ĵ������أ����ǽ���������̽����
������ͼ��裩
���������ͭ���Ǵ�����Ҳ�����뷴Ӧ����Ӧǰ�������ͻ�ѧ���ʲ��䣻
���������ͭ���뷴Ӧ������������Ӧǰ�������ͻ�ѧ���ʷ����˸ı䣻
���������ͭ�Ƿ�Ӧ�Ĵ�������Ӧǰ��________________��
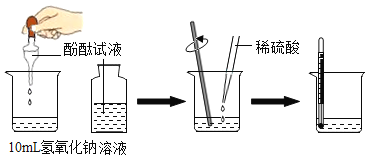
������ʵ�飩
����ƽ����![]() ����ͭ��ȡ
����ͭ��ȡ![]() �Ĺ���������Һ���Թ��У���������ʵ�飺
�Ĺ���������Һ���Թ��У���������ʵ�飺
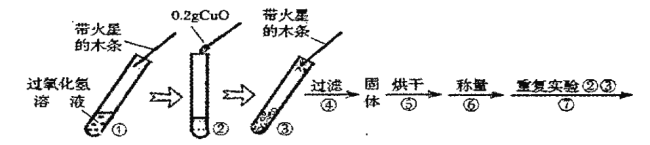
��1��������
��������� | ������ | ��������� | ���� |
��Һ�������ݷų��������ǵ�ľ����ȼ | ___________________ | ____________ | �����������������. |
��2������ٵ�Ŀ����______________________������ߵ�Ŀ��_______________��
��3����д����ʵ���з�Ӧ�Ļ�ѧ���ţ������֣�����ʽ______________________��