��Ŀ����
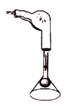
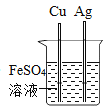
����Ŀ����ͼ��ʾ��ƿ����һö��ĥ��������������Һ©����װ��һ����Һ�壬ʵ��ǰU����Һ��b��c����ˮƽ��
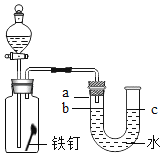
��1������Һ©���е�Һ����ˮ����Һ©������ע��һ����ˮ������������һ��ʱ���۲쵽U����Һ��b������a����c��֮�½���������֪ʶ��������������_____��
��2������Һ©�����Һ��ע��ƿ�ڣ�U��Һ����ֳ�b��c�ߵ�����Һ©���ڵ�Һ�������_____����Ӧ�Ļ�ѧ����ʽΪ_____��
���𰸡�����������������������ƿ����ѹ��С ϡ���� Fe+2HCl�TFeCl2+H2��
��������
��1������Һ©���е�Һ����ˮ����Һ©������ע��һ����ˮ������������һ��ʱ���۲쵽U����Һ��b������a����c��֮�½�������Ϊ����������������������ƿ����ѹ��С,�������������������������ƿ����ѹ��С��
��2������Һ©�����Һ��ע��ƿ�ڣ�U��Һ����ֳ�b��c�ߵ�������ƿ������������ӣ���Һ©���ڵ�Һ�������ϡ���ᣬϡ���������Ӧ�����Ȼ�����������������ƿ����ѹ������ϡ���
ϡ���������Ӧ�����Ȼ��������������ʷ�Ӧ�Ļ�ѧ����ʽдΪ��Fe+2HCl�TFeCl2+H2����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�