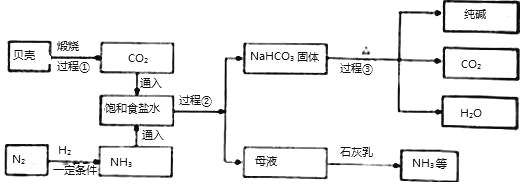
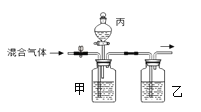
��Ŀ����
����Ŀ������������ʵ���ҳ��õ��Լ���ʵ����һ��������������Һ���Ȼ����Һ��Ӧ����ȡN2��N2�Ļ�ѧ����ʮ���ȶ�������һ������������H2���ֻ�������NH3��ת���ʺܵͣ�����ͼΪ��ȡ����NH3��װ�ã���ȡH2��װ������ȥ����
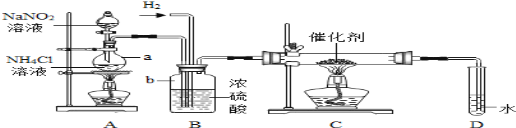
��1��ָ���������������a______��b______��
��2��Aװ���г���N2�����⣬����ˮ���Ȼ������ɣ�д����Ӧ�Ļ�ѧ����ʽ__________��
��3��Cװ�õ�Ӳ���Թ��з�����Ӧ�Ļ�ѧ����ʽΪ_____________
��4��д��ʵ������ȡH2�Ļ�ѧ����ʽ___________����ӦʱN2��H2�������������______,����װ������ʹ����ƽ�������ķ�ʽ��____________________
��5��Bװ�õ�������____________�������ٴ����㣩
��6�����������ܽ���ˮ�У�����Dװ��ȴû�в��÷�����װ�ã�С����Ϊװ��û�д���������__________
��7������װ�õ�ȱ����______________________
���𰸡���ƿ ����ƿ NaNO2+NH4Cl![]() N2��+2H2O+NaCl 3H2+N2
N2��+2H2O+NaCl 3H2+N2 ![]() 2NH3 Zn+H2SO4=ZnSO4+H2�� 14��3 ���Ʒ�Һ©���ĵμ��ٶ� ���ﰱ������������ ��Ϊ������ת���ʺܵͣ����ɵİ�������Ҳ���ǽ���D�Թܵ������к��н��ٵİ��� ת���ʵ�
2NH3 Zn+H2SO4=ZnSO4+H2�� 14��3 ���Ʒ�Һ©���ĵμ��ٶ� ���ﰱ������������ ��Ϊ������ת���ʺܵͣ����ɵİ�������Ҳ���ǽ���D�Թܵ������к��н��ٵİ��� ת���ʵ�
��������
��1��a����ƿ��b�Ǽ���ƿ��
��2��Aװ���г���N2�����⣬����ˮ���Ȼ������ɣ���Ӧ�Ļ�ѧ����ʽ NaNO2+NH4Cl![]() N2��+2H2O+NaCl��
N2��+2H2O+NaCl��
��3��Cװ�õ�Ӳ���Թ��У������͵����ڴ��������������ȷ�Ӧ���ɰ�������Ӧ�Ļ�ѧ����ʽΪ��3H2+N2![]() 2NH3��
2NH3��
��4��ʵ������ȡ��������п��ϡ���ᷴӦ��������п����������Ӧ�Ļ�ѧ����ʽΪ��Zn+H2SO4=ZnSO4+H2�����ɻ�ѧ����ʽΪ��3H2+N2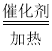 2NH3��֪��ӦʱN2��H2������������ǣ���14��2������2��3��=14��3������װ������ʹ����ƽ�������ķ�ʽ���ڸ�����װ���з�Һ©��������ͨ����Һ©���Ŀ���ʵ�ַ�Ӧ���ʵĿ��ƣ����Կ��Ʒ�Һ©���ĵμ��ٶȣ�
2NH3��֪��ӦʱN2��H2������������ǣ���14��2������2��3��=14��3������װ������ʹ����ƽ�������ķ�ʽ���ڸ�����װ���з�Һ©��������ͨ����Һ©���Ŀ���ʵ�ַ�Ӧ���ʵĿ��ƣ����Կ��Ʒ�Һ©���ĵμ��ٶȣ�
��5��B��Ũ����������Ǹ��������͵�����
��6�����������ܽ���ˮ�У�����Dװ��ȴû�в��÷�����װ�ã������ʾ��Ϣ��ת���ʺܵͣ�������������Ϊ������ת���ʺܵͣ����ɵİ�������Ҳ���ǽ���D�Թܵ������к��н��ٵİ�����
��7�����ݸ�������Ϣ��֪�����ַ���ת���ʵͣ����ܺĵȴ������ɣ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ������ͭ����һ�ֽ����Ļ�����ĩ����һ�ֽ���������þ������п�е�һ�֣������ⶨ����ɡ�
��ʵ�鲽�輰���ݣ�ȡ�û�����ĩ8.0 g�����ձ��У���140.0g14.0%��ϡ������Ĵμ��뵽���ձ��У���ַ�Ӧ���ʣ��������������ݼ�¼���£�
���� | 1 | 2 | 3 | 4 |
����ϡ���������/E | 35.0 | 35.0 | 35.0 | 35.0 |
ʣ����������/g | 6.8 | 5.6 | 4.4 | 4.2 |
ͨ�����㣨д��������̣������ȷ��0.1%������
��1���û�����ĩ��ͭ����������____________��
��2���û�����ĩ����һ����Ϊ���ֽ���____________��
��3�������μ��������ַ�Ӧ��������Һ�����ʵ����������Ƕ���____________��