��Ŀ����
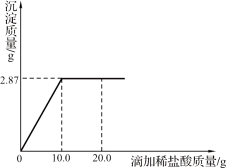
����Ŀ����5�֣�Ϊ�ⶨijAgNO3��Һ������������������������������Ϊ7.3%��ϡ������еζ�����ȡ����Һ50.0 g���ζ�������ͼ��ʾ��
���ζ���ӦΪ��AgNO3��HCl��AgCl����HNO3��Ag�����ԭ������Ϊ108��������������1λС����
��1��AgNO3����Է�������Ϊ��_____��
��2������AgNO3��Һ����������������
��3������ǡ����ȫ��Ӧʱ��������Һ����������������
���𰸡���1��170�� ��2��6.8% ��3��2.2%
��������
�����������1��AgNO3����Է�������=108+14+16��3=170��
��2�����û�ѧ����ʽ�����ݷ�Ӧ�������ȼ��ɼ������������������
��50.0 g����Һ��AgNO3������Ϊx����������Ϊa
AgNO3��HCl��AgCl����HNO3
170 143.5
x 2.87 g
143.5 x��170��2.87 g
x��![]() ��3. 4 g
��3. 4 g
a��![]() ��100%��6.8%
��100%��6.8%
��3����ǡ����ȫ��Ӧʱ��������Һ������HNO3������Ϊy����������Ϊb��
AgNO3��HCl��AgCl����HNO3
143.5 63
2.87 g y
143.5 y��63��2.87 g
y��![]() ��1.26 g
��1.26 g
ǡ����ȫ��Ӧʱ��Һ����50.0 g��10.0 g��2.87��57.13 g
b��![]() ��100%��2.2%
��100%��2.2%
����

 �������ͬ������ϵ�д�
�������ͬ������ϵ�д�