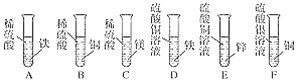
��Ŀ����
����Ŀ�����ǵ�����������벻����Դ��
(1)Ŀǰ����ʹ�õ�ȼ�ϴ�����Ի�ʯȼ�ϣ���______��ʯ�͡���Ȼ����,����______��(��������������������)��Դ��
(2)��ʯ���з�������͡����͵ȵĹ��̽�ʯ�͵ķ���,ԭ���Ǹ��ݸ����ʵ�_______��ͬ���ȵ�ԭ�ͽ��з���,��_______�仯;
(3)����5��18��.�Ϻ�����ȼ����(ѧ����Ȼ��ˮ�����Ҫ�ɷ���CH4)�Բɳɹ�����־���й��Ŀ���ʵ���Ѵ�������ǰ�С���ȼ��ȼ�յĻ�ѧ����ʽ��____________.
(4)�⻯þ(MgH2)�������Ϊ�����������Դ�ṩ�����ṩ��Դʱ��ˮ��Ӧ����������þ��һ�ֿ�ȼ�����壬��Ӧ�Ļ�ѧ����ʽ��________,ѡ���⻯þ��Ϊ��Դ�ĸ��ŵ���________.
���𰸡� ú �������� �е� ���� CH4+202![]() C02+H20 MgH2+2H20=Mg(0H)2+2H2 �� Я�����㣬ʹ�ð�ȫ������ȼ������Ⱦ��
C02+H20 MgH2+2H20=Mg(0H)2+2H2 �� Я�����㣬ʹ�ð�ȫ������ȼ������Ⱦ��
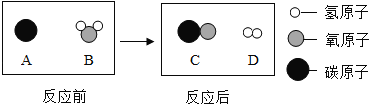
�����������⿼���˻�ʯȼ�ϼ����ۺ���������ѧ�仯�������仯���б�����ѧ����ʽ����д�ȡ�
��1��ú��ʯ�ͺ���Ȼ��������ʯȼ�ϣ������ڲ���������Դ��
��2������ʯ���и���ֵķе㲻ͬ����ʯ���з�������͡�ú�ͺͲ��͵ȣ����������û�����������ɣ����������仯��
��3������ȼ�����ɶ�����̼��ˮ���䷴Ӧ�Ļ�ѧ����ʽΪ��CH4 + 2O2![]() CO2 +2 H2O
CO2 +2 H2O
��4��MgH2���Ժ�ˮ��Ӧ����������þ���������÷�Ӧ�Ļ�ѧ����ʽΪ��MgH2+2H2O�TMg��OH��2+2H2�����⻯þ��Ϊ��Դ���ŵ���Я�����㣬ʹ�ð�ȫ������Ⱦ��

����Ŀ��ʯ��ʯ�������������Ȼ��Ƶȶ��ֻ�����Ʒ��ԭ�ϡ�ij�о���ѧϰС��Ϊ�˲ⶨ���ؿ�ɽʯ��ʯ��̼��Ƶ�����������ȡ���˿�ʯ��Ʒ����ȡϡ����200g��ƽ���ֳ�4�ݣ�����ʵ�飬������£�
ʵ �� | 1 | 2 | 3 | 4 |
������Ʒ����/g | 5 | 10 | 15 | 20 |
����CO2������/g | 1.76 | 3.52 | 4.4 | m |
(1)�ڼ��η�Ӧ�п�ʯ��ʣ�ࣿ_________��
(2)����mֵ��________��
(3)�Լ�������ʯ��ʯ��̼��Ƶ���������Ϊ_________��