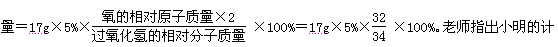��Ŀ����
��11�֣�����֪ʶ������һ����Ҫ��ѧϰ������ͼΪijУ��ѧѧϰС���С��ͬѧ���Ƶ����Ļ�ѧ��������ʾ��ͼ��������ش��������⣺
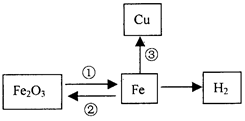
(1)��Ӧ���п��� �� (�ѧʽ)����ԭ����
��д����¯�з����ķ�Ӧ�ٵĻ�ѧ����
ʽ�� �� ��
д����Ӧ�۵Ļ�ѧ����ʽ �� ��
�Ƿ�Ӧ�ڵ���ÿ���д����ĸ�����ʴ��Ϊ��ֹ�÷�Ӧ�ķ��������dz������ڸ�������Ϳˢ�����������������ȷ����������ַ����Ĺ�ͬԭ������ֹ����
�� �� �Ӵ���
�ȸû�ѧѧϰС��Ϊ�˷������������ĺ���������������ʵ���о���ȡ12g������10%�����ᷴӦ���������˼�������������ų����������Ĺ�ϵͼ��������ͼ������˵�������������ʲ�����ˮ���������ᡢ����ͭ��Һ��Ӧ����
������ȫ��Ӧ��ȥ���������Ϊ �� g��
��������������������Ϊ���٣���д��������̣����������С�����һλ��
��
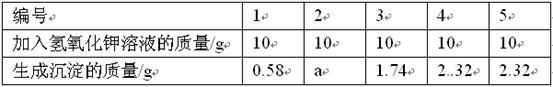
��5��ijͬѧ�г������¼���ʽ��
����������պ÷�Ӧ��ȫʱ������Һ����= (146+12-a)g
С���Ըü���ʽ��������ɣ���ָ�����еĴ��� ��
��
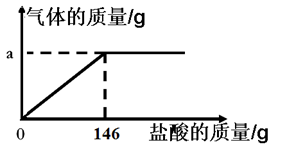
��6��С���������һ��ʵ�鷽������12g��������20%������ͭ��Һ��Ӧ���������������ĺ��������㻭����������ͭ��Һ��������������仯��ϵ�����ߡ�


(1)��Ӧ���п��� �� (�ѧʽ)����ԭ����
��д����¯�з����ķ�Ӧ�ٵĻ�ѧ����
ʽ�� �� ��
д����Ӧ�۵Ļ�ѧ����ʽ �� ��
�Ƿ�Ӧ�ڵ���ÿ���д����ĸ�����ʴ��Ϊ��ֹ�÷�Ӧ�ķ��������dz������ڸ�������Ϳˢ�����������������ȷ����������ַ����Ĺ�ͬԭ������ֹ����
�� �� �Ӵ���
�ȸû�ѧѧϰС��Ϊ�˷������������ĺ���������������ʵ���о���ȡ12g������10%�����ᷴӦ���������˼�������������ų����������Ĺ�ϵͼ��������ͼ������˵�������������ʲ�����ˮ���������ᡢ����ͭ��Һ��Ӧ����
������ȫ��Ӧ��ȥ���������Ϊ �� g��
��������������������Ϊ���٣���д��������̣����������С�����һλ��
��
��5��ijͬѧ�г������¼���ʽ��
����������պ÷�Ӧ��ȫʱ������Һ����= (146+12-a)g
С���Ըü���ʽ��������ɣ���ָ�����еĴ��� ��
��
��6��С���������һ��ʵ�鷽������12g��������20%������ͭ��Һ��Ӧ���������������ĺ��������㻭����������ͭ��Һ��������������仯��ϵ�����ߡ�
(1)C��H2��CO��0.5�֣�

��2�� ����
Fe2O3+3CO====2Fe+3CO2
Fe+CuSO4==FeSO4+Cu����Fe+CuCl2==FeCl2+Cu�� Fe+Cu��NO3��2==Fe��NO3��2+Cu ��
(3)������ˮ����������ˮ��
��4����146��0.5�֣�
�ڣ���4��
�⣺�������ᷴӦ�����ĵ�����Ϊx�� 0.5��
 Fe + 2HCl �� FeCl2 + H2��(1��)
Fe + 2HCl �� FeCl2 + H2��(1��)
56 73
x 146 g��10%="14.6" g
x =" 11.2g" 0.5��
������������������Ϊ=��11.2g��12g����100% = 93.3% 1��
��������������������Ϊ93.3%��
��5����Һ��������������������ʣ�����ʵ����������Ի�Ҫ��ȥ���ʵ�����
��6��ץס��㡢�۵㼰�������Ƹ��֣�����Ҫ��ע��Щ�㣬ץס��㡢�۵㼰�����������֣���һ�������֣��������ξ�Ϊֱ�ߣ�ƫ��̫���֣�����㡢�۵㼰��������û�д��������£����ò���λ����ֻ����ǰ�벿�֣�����ȷ���Ŀ��ʵ��۷֡���2�֣�

��2�� ����
Fe2O3+3CO====2Fe+3CO2
Fe+CuSO4==FeSO4+Cu����Fe+CuCl2==FeCl2+Cu�� Fe+Cu��NO3��2==Fe��NO3��2+Cu ��
(3)������ˮ����������ˮ��
��4����146��0.5�֣�
�ڣ���4��
�⣺�������ᷴӦ�����ĵ�����Ϊx�� 0.5��
 Fe + 2HCl �� FeCl2 + H2��(1��)
Fe + 2HCl �� FeCl2 + H2��(1��)56 73
x 146 g��10%="14.6" g
 | |||
 | |||
x =" 11.2g" 0.5��
������������������Ϊ=��11.2g��12g����100% = 93.3% 1��
��������������������Ϊ93.3%��
��5����Һ��������������������ʣ�����ʵ����������Ի�Ҫ��ȥ���ʵ�����
��6��ץס��㡢�۵㼰�������Ƹ��֣�����Ҫ��ע��Щ�㣬ץס��㡢�۵㼰�����������֣���һ�������֣��������ξ�Ϊֱ�ߣ�ƫ��̫���֣�����㡢�۵㼰��������û�д��������£����ò���λ����ֻ����ǰ�벿�֣�����ȷ���Ŀ��ʵ��۷֡���2�֣�
��������1�����õĻ�ԭ����̼��һ����̼���������֣�
��2����¯��������һ����̼��ԭ�����������û�ͭ������ͭ������Һ��Ӧ��
��3�������������������������ˮͬʱ�Ӵ���
��4����ͼ��ֱ�ӿ�����ȫ��Ӧ����������Һ�������������������ᷴӦ�ķ���ʽ�������������������ɵõ��μӷ�Ӧ���������������������������������������
��5����Ӧ����Һ����=�μӷ�Ӧ�Ĺ�������+Һ������-��������-����������
��6����ͼ����������ҵ����ؼ����ҵ��յ㣬����������ͭǡ����ȫ��Ӧʱ���ĵ�����ͭ��Һ����������ʣ������������
�⣺��1����������ԭ����C��H2��CO�������ڢٴ�����дһ����
��2����¯������һ�����ӵĹ��̣�������Ҫ�����ý�̿��������Ӧ���ɵ�һ����̼����������ʯ�л�ԭ����������ʽΪFe2O3+3CO====2Fe+3CO2����ͭ������Һ��Ӧ�ɰ�ͭ�û��������緽��ʽ��Fe+CuSO4�TFeSO4+Cu��Fe+CuCl2�TFeCl2+Cu�� Fe+Cu��NO3��2�TFe��NO3��2+Cu��
��3�������������������������ˮͬʱ�Ӵ������Է�ֹ�������ԭ������ʹ������������ˮ����������ˮ����
��4������ͼ��֪����ȫ��Ӧʱ���ĵ�����������146g��
�ڽ⣺�������ᷴӦ����������Ϊx��
Fe+2HCl=FeCl2+H2��
56 73
x 146 g��10%="14.6" g

��ã�x=11.2g
��������������������
 ��100%=93.3%
��100%=93.3%
��������������������Ϊ93.3%��
��5����Ϊ��Һ��������������������ʣ�����ʵ����������Ի�Ҫ��ȥ���ʵ�������
��6����������ͭ��δ��Ӧʱ�������������Ϊ12g�������Ӧ��������12��ʼ����
Ȼ���ҹյ㣬����������ͭǡ����ȫ��Ӧʱ���ĵ�����ͭ��Һ����������������
����������ͭǡ����ȫ��Ӧʱ��������ͭ��ҺΪy������ͭΪz
Fe+CuSO4 �TFeSO4 +Cu
56 160 64
11.2g 20%?y z

y=160g

z=12.8g
12g����������������12g-11.2g=0.8g
������������ͭǡ����ȫ��Ӧʱ��������Ϊ��12.8g+0.8g=13.6g
���յ㴦������ֵΪ160��������ֵΪ13.6��Ȼ��ѹյ������������������ڹյ��һ��ƽ����x���ֱ�ߣ��͵õ�����Ҫ������ͼ��
�ʴ�Ϊ����1��CO����2��Fe2O3+3CO 2Fe+3CO2��Fe+CuSO4�TFeSO4+Cu��
2Fe+3CO2��Fe+CuSO4�TFeSO4+Cu��
��3��������ˮ��
��4����146����93.3%��
��5����Һ����������ʣ�����ʵ����������Ի�Ҫ��ȥ���ʵ�������
��6��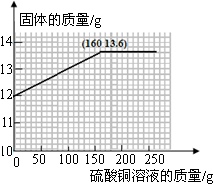
�������ۺ��Խ�ǿ����Ŀ������������϶࣬�漰��֪ʶ��ܹ㣬�����ѶȽϴ����Ŀ��
��2����¯��������һ����̼��ԭ�����������û�ͭ������ͭ������Һ��Ӧ��
��3�������������������������ˮͬʱ�Ӵ���
��4����ͼ��ֱ�ӿ�����ȫ��Ӧ����������Һ�������������������ᷴӦ�ķ���ʽ�������������������ɵõ��μӷ�Ӧ���������������������������������������
��5����Ӧ����Һ����=�μӷ�Ӧ�Ĺ�������+Һ������-��������-����������
��6����ͼ����������ҵ����ؼ����ҵ��յ㣬����������ͭǡ����ȫ��Ӧʱ���ĵ�����ͭ��Һ����������ʣ������������
�⣺��1����������ԭ����C��H2��CO�������ڢٴ�����дһ����
��2����¯������һ�����ӵĹ��̣�������Ҫ�����ý�̿��������Ӧ���ɵ�һ����̼����������ʯ�л�ԭ����������ʽΪFe2O3+3CO====2Fe+3CO2����ͭ������Һ��Ӧ�ɰ�ͭ�û��������緽��ʽ��Fe+CuSO4�TFeSO4+Cu��Fe+CuCl2�TFeCl2+Cu�� Fe+Cu��NO3��2�TFe��NO3��2+Cu��
��3�������������������������ˮͬʱ�Ӵ������Է�ֹ�������ԭ������ʹ������������ˮ����������ˮ����
��4������ͼ��֪����ȫ��Ӧʱ���ĵ�����������146g��
�ڽ⣺�������ᷴӦ����������Ϊx��
Fe+2HCl=FeCl2+H2��
56 73
x 146 g��10%="14.6" g

��ã�x=11.2g
��������������������
 ��100%=93.3%
��100%=93.3%��������������������Ϊ93.3%��
��5����Ϊ��Һ��������������������ʣ�����ʵ����������Ի�Ҫ��ȥ���ʵ�������
��6����������ͭ��δ��Ӧʱ�������������Ϊ12g�������Ӧ��������12��ʼ����
Ȼ���ҹյ㣬����������ͭǡ����ȫ��Ӧʱ���ĵ�����ͭ��Һ����������������
����������ͭǡ����ȫ��Ӧʱ��������ͭ��ҺΪy������ͭΪz
Fe+CuSO4 �TFeSO4 +Cu
56 160 64
11.2g 20%?y z

y=160g

z=12.8g
12g����������������12g-11.2g=0.8g
������������ͭǡ����ȫ��Ӧʱ��������Ϊ��12.8g+0.8g=13.6g
���յ㴦������ֵΪ160��������ֵΪ13.6��Ȼ��ѹյ������������������ڹյ��һ��ƽ����x���ֱ�ߣ��͵õ�����Ҫ������ͼ��
�ʴ�Ϊ����1��CO����2��Fe2O3+3CO
 2Fe+3CO2��Fe+CuSO4�TFeSO4+Cu��
2Fe+3CO2��Fe+CuSO4�TFeSO4+Cu����3��������ˮ��
��4����146����93.3%��
��5����Һ����������ʣ�����ʵ����������Ի�Ҫ��ȥ���ʵ�������
��6��

�������ۺ��Խ�ǿ����Ŀ������������϶࣬�漰��֪ʶ��ܹ㣬�����ѶȽϴ����Ŀ��

��ϰ��ϵ�д�
 ����Ӣ��ϵ�д�
����Ӣ��ϵ�д�
�����Ŀ