��Ŀ����
���ϣ�����Ҫ��������ס�������벻����ˮ���������������ϼ�û��ˮ��û�п�������ѧ�ҷ��������ɳ���к��зḻ�ĺ��������ɷ�ΪTiO2��Fe2O3�Ļ����,����Щ��ʯ����CO����ԭ���������Ѻ���,����������ԭ�����Եõ���ˮ������Щ��ʯͨ�磬�����Դ��з����������
�١����������д�����Ȼ�������������ȶ��ֽ����������������������Au��������Ͻ����л���ijЩ����������Ͻ������������������г�ַ�Ӧ��Ȼ����ˣ�ʣ��Ĺ����п��Ի��յõ��Ľ�������� ����������
A��Fe�� B��Au��Fe C��Ag��Au D��Fe��Ag
�ڡ��ݿ�ѧ��Ԥ�⣬����������������Ű���ֵĺ���He-3������ԭ�Ӻ���������Ϊ2��������Ϊ1��ÿ�ٶֺ���He-3���˾۱����ͷŵ������൱��Ŀǰ����һ�����ĵ����������й��ں���He-3��Ԫ�ص�˵����ȷ���� �� ��
�ۡ����϶�һ�š�����Ļ���ƽ�����װ��Һ̬�£���A��ʾ���������⣨H2O2�������ǻ��ʱ�ķ�Ӧ����ʽΪ��2H2O2+A==N2+4H2O �����£�A���Ļ�ѧʽΪ�� ��
A��N2H2 B��N2H4O2 C��NH2 D��N2H4
�ܡ�������������������������������Ʒ (��ס����ס�)���⣬ԭ���ǣ�
��
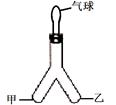
�ݡ�������CO����ԭTiO2��Fe2O3�Ļ�������������Ѻ�����װ����ͼ��ʾ���Իش�
�� д��A�з�����Ӧ��ѧ����ʽ������һ���� ��
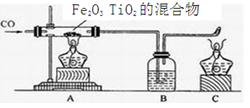
����CO��ԭ�����������Ѻ����Ļ������ü������������������ʷֿ� ��
�Ǿ�ʵ�����˳���������λͬѧ�����˷��硣С����Ϊ��ͨһ��ʱ��CO�ſգ�Ȼ���ٵ�ȼC��A�еľƾ��ƣ�С����Ϊ���������ã����������� ��
С����Ϊ�ȵ�ȼC��A�еľƾ��ƣ�Ȼ����ͨCO��С����������ԣ�����������
��ʦ��Ϊ���ǵķ����������һ�������������������λͬѧ������ǵ����ѣ���İ취��
�١����������д�����Ȼ�������������ȶ��ֽ����������������������Au��������Ͻ����л���ijЩ����������Ͻ������������������г�ַ�Ӧ��Ȼ����ˣ�ʣ��Ĺ����п��Ի��յõ��Ľ�������� ����������
A��Fe�� B��Au��Fe C��Ag��Au D��Fe��Ag
�ڡ��ݿ�ѧ��Ԥ�⣬����������������Ű���ֵĺ���He-3������ԭ�Ӻ���������Ϊ2��������Ϊ1��ÿ�ٶֺ���He-3���˾۱����ͷŵ������൱��Ŀǰ����һ�����ĵ����������й��ں���He-3��Ԫ�ص�˵����ȷ���� �� ��
�ۡ����϶�һ�š�����Ļ���ƽ�����װ��Һ̬�£���A��ʾ���������⣨H2O2�������ǻ��ʱ�ķ�Ӧ����ʽΪ��2H2O2+A==N2+4H2O �����£�A���Ļ�ѧʽΪ�� ��
A��N2H2 B��N2H4O2 C��NH2 D��N2H4
�ܡ�������������������������������Ʒ (��ס����ס�)���⣬ԭ���ǣ�
��
�ݡ�������CO����ԭTiO2��Fe2O3�Ļ�������������Ѻ�����װ����ͼ��ʾ���Իش�
�� д��A�з�����Ӧ��ѧ����ʽ������һ���� ��

����CO��ԭ�����������Ѻ����Ļ������ü������������������ʷֿ� ��
�Ǿ�ʵ�����˳���������λͬѧ�����˷��硣С����Ϊ��ͨһ��ʱ��CO�ſգ�Ȼ���ٵ�ȼC��A�еľƾ��ƣ�С����Ϊ���������ã����������� ��
С����Ϊ�ȵ�ȼC��A�еľƾ��ƣ�Ȼ����ͨCO��С����������ԣ�����������
��ʦ��Ϊ���ǵķ����������һ�������������������λͬѧ������ǵ����ѣ���İ취��
��C ��D ��D ������ �����ϼ�û��ˮ��û������
�ݣ�1��3CO + Fe2O3 3CO2+ 2Fe ��2���ô������� ��3������CO��ɢ����������ɿ�����Ⱦ ��ʹCO������������ը �ȵ�ȼc���ľƾ�����ͨCO�ž�װ���ڿ������ٵ�ȼA���ƾ���
3CO2+ 2Fe ��2���ô������� ��3������CO��ɢ����������ɿ�����Ⱦ ��ʹCO������������ը �ȵ�ȼc���ľƾ�����ͨCO�ž�װ���ڿ������ٵ�ȼA���ƾ���
�ݣ�1��3CO + Fe2O3
 3CO2+ 2Fe ��2���ô������� ��3������CO��ɢ����������ɿ�����Ⱦ ��ʹCO������������ը �ȵ�ȼc���ľƾ�����ͨCO�ž�װ���ڿ������ٵ�ȼA���ƾ���
3CO2+ 2Fe ��2���ô������� ��3������CO��ɢ����������ɿ�����Ⱦ ��ʹCO������������ը �ȵ�ȼc���ľƾ�����ͨCO�ž�װ���ڿ������ٵ�ȼA���ƾ�������������� ���ݽ������˳���жϣ��������ᷴӦ�������ͽ�Ӧ�����ʣ��Ĺ����п��Ի��յõ��Ľ���Ϊ���ͽ𣬹�ѡC��
�� ��ԭ���У�����������=�����������Ӧ����2����A��C�������ԭ������=������+��������Ӧ����3����B�������ѡ��D��
�� ���������غ㶨�ɣ��ڻ�ѧ��Ӧǰ��ԭ�ӵĸ������䣬�õ�A�Ļ�ѧʽΪN2H4����ѡD���� ������������������ˮ��ͬ���õĽ�������������ϼ�û��ˮ����û�����������������ϵ�����Ʒ�������⡣
�� ��������ԭ������һ����̼Ϊ��ԭ���������ӿ�ʯ�л�ԭ��������ѧ����ʽΪ��
3CO + Fe2O3
 3CO2+ 2Fe
3CO2+ 2Fe �Ʒ�������Ӧ��ѡ��������������ѡ��ѧ���������������Ա���������������Ӧѡ�ô��������ķ��������Ѻ����ֿ���
��3����Ϊһ����̼�ж�����������д���������Ⱦ����������С�µ��������ã���С���ȵ�ȼ�ƾ��ƣ���ʹCO������������ը�����С��������Ҳ���ã���Ҫ��ֹ��ը��Ҫ���������Ⱦ��Ӧ�ȵ�ȼc���ľƾ�����ͨCO�ž�װ���ڿ������ٵ�ȼA���ƾ�����
���������ڴ������⣬Ҫ��ѧ�����й����Ļ�ѧ���ʡ�ұ��������ʵ����̣��Լ�ʵ���е�ע�������֪ʶ�ۺ���һ��

��ϰ��ϵ�д�
 Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�
�����Ŀ