题目内容
图图同学为测定某石灰石中碳酸钙的质量分数(杂质不与酸反应),向6.0g石灰石样品中逐滴加入稀盐酸至不再产生气泡为止,共生成二氧化碳气体1.1L(该条件下二氧化碳气体的密度为2g/L)。试计算:
(1)该反应生成二氧化碳的质量为 g;(精确到0.1g)
(2)该石灰石样品中碳酸钙的质量分数为多少?(写出计算过程,结果精确至0.1%)
(3)若要计算上述反应所消耗盐酸溶液的质量,题中还缺少的一个数据是 。
(1)该反应生成二氧化碳的质量为 2.2 g;(精确到0.1g) 1分
(2)解:设碳酸钙的质量为x
2HCl + CaCO3= CaCl2+ H2O+CO2↑
 100 44
100 44
 X 2.2
X 2.2
|


 X 2.2
X 2.2
X=5g




 CaCO3%=
CaCO3%=

 =83.3%
=83.3%
(3)题中还缺少的一个数据是 参加反应的稀盐酸的质量 。 1分

练习册系列答案
相关题目
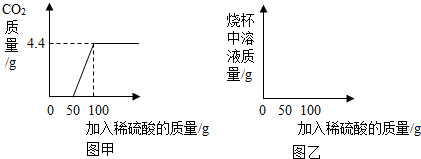
 (2008?荆州)草木灰是农村常用的一种钾肥,其中只有其有效成分K2CO3能溶于水.实验中学化学兴趣小组的同学为了测定某草木灰中K2CO3的含量,称取该草木灰100g用足量的水将其溶解.经过滤、洗涤(洗液并入滤液中)后,将滤液蒸发至80.4g时停止加热并冷至室温,再向其中逐滴加入稀盐酸,同时将生成的CO2用足量的石灰乳[Ca(OH)2]吸收.加入盐酸的质量m与石灰乳中增加的质量△m的关系如图所示.
(2008?荆州)草木灰是农村常用的一种钾肥,其中只有其有效成分K2CO3能溶于水.实验中学化学兴趣小组的同学为了测定某草木灰中K2CO3的含量,称取该草木灰100g用足量的水将其溶解.经过滤、洗涤(洗液并入滤液中)后,将滤液蒸发至80.4g时停止加热并冷至室温,再向其中逐滴加入稀盐酸,同时将生成的CO2用足量的石灰乳[Ca(OH)2]吸收.加入盐酸的质量m与石灰乳中增加的质量△m的关系如图所示.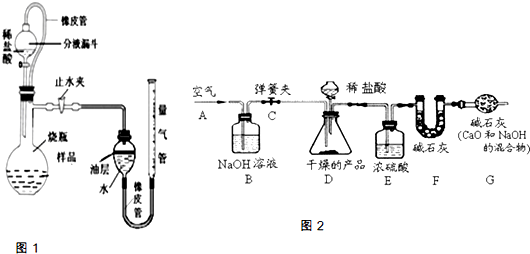
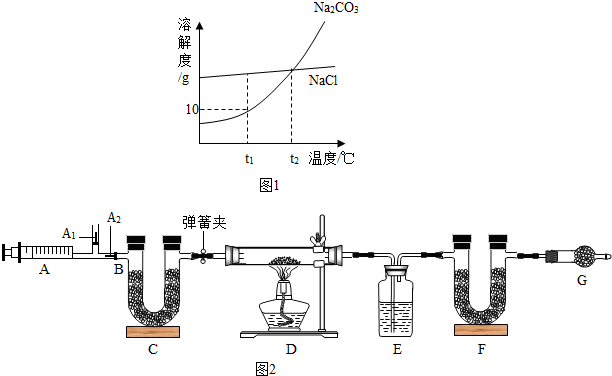
 Na2CO3+CO2↑+H2O (3)图中B处为两个单向阀:推注射器时A1关闭,A2处打开;拉注射器时,A1打开进空气,A2关闭.
Na2CO3+CO2↑+H2O (3)图中B处为两个单向阀:推注射器时A1关闭,A2处打开;拉注射器时,A1打开进空气,A2关闭.