��Ŀ����
�������̼����ɹ�Ρ�������ġ����ָNa2CO3�����Ρ���ָNaCl��
��һ��Na2CO3��NaCl���ܽ��������ͼ1��ʾ������ͼ�ش�
��t1��ʱNa2CO3���ܽ��Ϊ
t2��ʱNa2CO3���ܽ��
�ڡ������̼��ԭ��������Na2CO3���ܽ�����¶Ƚ��Ͷ�
�ۡ�����ɹ�Ρ�������
A���紵��ɹ��ʹ�ܼ����� B�������¶ȣ�ʹNaCl�ܽ������
�������ҹ��ຣ�������õ�����Ȼ�����̼���Ƶľ��壬��ɿɱ�ʾΪaNa2CO3?bNaHCO3?cH2O��a��b��cΪ��������ȣ���ij��ѧ����С�����Ȼ��ijɷֽ���̽����
С��ͬѧΪ�ⶨ����ɣ���ȡ����Ȼ����Ʒ16.6g������ͼ2ʵ�飺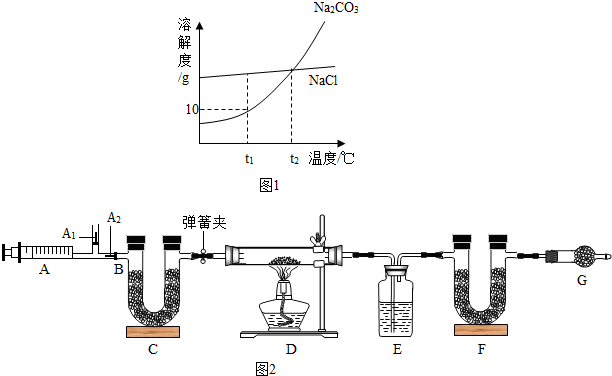
���������ϡ�
��1��̼���ƱȽ��ȶ�������ʱ���ֽ⣻
��2��2NaHCO3
Na2CO3+CO2��+H2O ��3��ͼ��B��Ϊ����������ע����ʱA1�رգ�A2������ע����ʱ��A1��������A2�رգ�
��ʵ�鲽�衿
����װ��װ�ã���������� �ڷ�������ע���� �۳���E��F�������ܹرյ��ɼУ�����D���Թ�ֱ����Ӧ���ٽ��� �ݴ��ɼУ��ٴη�����������ע���� ���ٴγ���E��F��������
������̽����
��1��E�е�ҩƷΪ
��2��C��F��G��װ�м�ʯ�ң�CaO��NaOH�Ĺ����������C��������
��3��ʵ�鲽�������ܷ�ߵ�
��4�����±���16.6g��Ȼ���нᾧˮ������Ϊ
��һ��Na2CO3��NaCl���ܽ��������ͼ1��ʾ������ͼ�ش�
��t1��ʱNa2CO3���ܽ��Ϊ
10
10
g��t2��ʱNa2CO3���ܽ��
�T
�T
NaCl���ܽ�ȣ������������������=�����ڡ������̼��ԭ��������Na2CO3���ܽ�����¶Ƚ��Ͷ�
��С
��С
���������С�����䡱�����ۡ�����ɹ�Ρ�������
A
A
������ţ��ķ�����ʹNaCl����������A���紵��ɹ��ʹ�ܼ����� B�������¶ȣ�ʹNaCl�ܽ������
�������ҹ��ຣ�������õ�����Ȼ�����̼���Ƶľ��壬��ɿɱ�ʾΪaNa2CO3?bNaHCO3?cH2O��a��b��cΪ��������ȣ���ij��ѧ����С�����Ȼ��ijɷֽ���̽����
С��ͬѧΪ�ⶨ����ɣ���ȡ����Ȼ����Ʒ16.6g������ͼ2ʵ�飺

���������ϡ�
��1��̼���ƱȽ��ȶ�������ʱ���ֽ⣻
��2��2NaHCO3
| ||
��ʵ�鲽�衿
����װ��װ�ã���������� �ڷ�������ע���� �۳���E��F�������ܹرյ��ɼУ�����D���Թ�ֱ����Ӧ���ٽ��� �ݴ��ɼУ��ٴη�����������ע���� ���ٴγ���E��F��������
������̽����
��1��E�е�ҩƷΪ
Ũ����
Ũ����
��E������������ˮ����
����ˮ����
����2��C��F��G��װ�м�ʯ�ң�CaO��NaOH�Ĺ����������C��������
��ȥ�����еĶ�����̼��ˮ��������������
��ȥ�����еĶ�����̼��ˮ��������������
��F���������������ɵĶ�����̼
�������ɵĶ�����̼
��G����������ֹ�����еĶ�����̼��ˮ����������������뵽F�У�Ӱ�������̼�����IJⶨ
��ֹ�����еĶ�����̼��ˮ����������������뵽F�У�Ӱ�������̼�����IJⶨ
����3��ʵ�鲽�������ܷ�ߵ�
����
����
����ܡ����ܡ������������в���ݵIJ�����������õ�̼��������������ƫС
ƫС
���ƫ����ƫС��������Ӱ�족�����ò�������ע����ʱ������Ŀ����ʹ���ɵĶ�����̼��ˮ�������ճ��
ʹ���ɵĶ�����̼��ˮ�������ճ��
��4�����±���16.6g��Ȼ���нᾧˮ������Ϊ
1.8
1.8
g��Na2CO3������Ϊ10.6
10.6
g������Ȼ��Ļ�ѧʽ��a��b��c=2��1��2
2��1��2
��| ��Ӧǰ | ��Ӧ�� |
| E������Ϊ100.0g | E������Ϊ102.25g |
| F������Ϊ50.0g | F������Ϊ51.1g |
�����������ܽ�����ߵ����弰�ܽ�����¶ȱ仯��Ӱ������ɸò��ֵڣ�һ��С��Ľ�𣻸���Ũ���ᡢ��ʯ�ҵ����ʷ����ж�ʵ������е���ط�������⣬���ݾ�������ݿ��Խ�����ط���ļ��㣬�Ӷ��ó���ȷ�Ľ��ۣ�
����⣺��һ���ٴ��ܽ�����߿��Եó�t1��ʱNa2CO3���ܽ��Ϊ10g��t2��ʱNa2CO3���ܽ����NaCl���ܽ����ȣ�
�ڴ��ܽ�����߿��Եó�̼���Ƶ��ܽ�����¶ȵ����߶��������¶Ƚ��Ͷ���С��
���Ȼ��Ƶ��ܽ�����¶ȱ仯Ӱ�첻��Ӧ��ͨ�������ķ����õ��Ȼ��ƾ��壬�������¶Ƚϸߣ������Ͽ죬��ѡA��
��������1��Eװ�õ�����������ˮ������Ũ����������ˮ���������Ũ���ᣨŨH2SO4����
E������������ˮ�������������ˮ������
��2��C�������dz�ȥ�����еĶ�����̼��ˮ�������������壮�����ȥ�����еĶ�����̼��ˮ�������������壮
F���������������ɵĶ�����̼������������ɵĶ�����̼��
G�����ã���ֹ�����еĶ�����̼��ˮ����������������뵽F�У�Ӱ�������̼�����IJⶨ��
��3������ߵ�˳���������ƫ�ߣ�������ܣ�
�������в���ݵIJ��������ɵĶ�����̼��ˮ���ܱ�������գ�ʹ�ý��ƫС�����ƫС��
�ò�������ע����ʱ������Ŀ����ʹ���ɵĶ�����̼��ˮ�������ճ�֣����ʹ���ɵĶ�����̼��ˮ�������ճ�֣�
��4�����ݱ��е����ݿ�֪����Ӧ�����ɶ�����̼������Ϊ51.1g-50g=1.1g����̼�����Ƶ�����Ϊx���ֽ����ɵ�ˮ������Ϊy�����ݻ�ѧ����ʽ
2NaHCO3
Na2CO3��+CO2��+H2O
168 44 18
x 1.1g y
���ݣ�
=
���x=4.2g��
���ݣ�
=
���y=0.45g������Ȼ���к���ˮ������Ϊ102.25g-100.0g-0.45g=1.8g
��Ȼ���к���̼���Ƶ�����Ϊ��16.6g-4.2g-1.8g=10.6g
������Ȼ�����ɿ�֪��
=
=
����a��b��c=2��1��2�����2��1��2��
�ʴ�Ϊ����һ����10��=���ڼ�С����A����������1��Ũ�������ˮ��������2����ȥ�����еĶ�����̼��ˮ�������������壻�������ɵĶ�����̼����ֹ�����еĶ�����̼��ˮ����������������뵽F�У�Ӱ�������̼�����IJⶨ����3�����ܣ�ƫС��ʹ���ɵĶ�����̼��ˮ�������ճ�֣���4��1.8��10.6��2��1��2��
�ڴ��ܽ�����߿��Եó�̼���Ƶ��ܽ�����¶ȵ����߶��������¶Ƚ��Ͷ���С��
���Ȼ��Ƶ��ܽ�����¶ȱ仯Ӱ�첻��Ӧ��ͨ�������ķ����õ��Ȼ��ƾ��壬�������¶Ƚϸߣ������Ͽ죬��ѡA��
��������1��Eװ�õ�����������ˮ������Ũ����������ˮ���������Ũ���ᣨŨH2SO4����
E������������ˮ�������������ˮ������
��2��C�������dz�ȥ�����еĶ�����̼��ˮ�������������壮�����ȥ�����еĶ�����̼��ˮ�������������壮
F���������������ɵĶ�����̼������������ɵĶ�����̼��
G�����ã���ֹ�����еĶ�����̼��ˮ����������������뵽F�У�Ӱ�������̼�����IJⶨ��
��3������ߵ�˳���������ƫ�ߣ�������ܣ�
�������в���ݵIJ��������ɵĶ�����̼��ˮ���ܱ�������գ�ʹ�ý��ƫС�����ƫС��
�ò�������ע����ʱ������Ŀ����ʹ���ɵĶ�����̼��ˮ�������ճ�֣����ʹ���ɵĶ�����̼��ˮ�������ճ�֣�
��4�����ݱ��е����ݿ�֪����Ӧ�����ɶ�����̼������Ϊ51.1g-50g=1.1g����̼�����Ƶ�����Ϊx���ֽ����ɵ�ˮ������Ϊy�����ݻ�ѧ����ʽ
2NaHCO3
| ||
168 44 18
x 1.1g y
���ݣ�
| 168 |
| x |
| 44 |
| 1.1g |
���ݣ�
| 44 |
| 18 |
| 1.1g |
| y |
��Ȼ���к���̼���Ƶ�����Ϊ��16.6g-4.2g-1.8g=10.6g
������Ȼ�����ɿ�֪��
| 106a |
| 10.6 |
| 84b |
| 4.2 |
| 18c |
| 1.8 |
�ʴ�Ϊ����һ����10��=���ڼ�С����A����������1��Ũ�������ˮ��������2����ȥ�����еĶ�����̼��ˮ�������������壻�������ɵĶ�����̼����ֹ�����еĶ�����̼��ˮ����������������뵽F�У�Ӱ�������̼�����IJⶨ����3�����ܣ�ƫС��ʹ���ɵĶ�����̼��ˮ�������ճ�֣���4��1.8��10.6��2��1��2��
���������������ܽ�����ߵ����壬�ܹ����������������غ㶨���Լ�������ѧ����ʽ���е���ؼ��㣬Ҫ����������ʵ����ʺ�ʵ��Ŀ�ģ�ֻ���������ܶ�����������ȷ���жϣ�

��ϰ��ϵ�д�
�����Ŀ
�±��зֱ���̼���ƺ��Ȼ��Ʋ�ͬ�¶��µ��ܽ��
�ҹ��������μ�����������д����Ĵ�����Ȼ��ƣ������ũ�����̼����ɹ�Σ�
��̼���ƺ��Ȼ��Ʋ�ͬ�¶��µ��ܽ�ȣ���ش�
��1��10��ʱ��̼���Ʊ��Ȼ��Ƶ��ܽ�� ��
��2������ͼ�����ݣ������̼��ԭ���� ��
��3��80��ʱ����100gˮ���Ƴ�̼���Ƶı�����Һ�����������������ǣ���ȷ������0.1%�� �����ø���Һ��������ʯ��ˮ��Ӧ���ɵ��������ƣ���ȷ�������� g��
| �¶ȡ� | 0 | 10 | 20 | 30 | 40 | 60 | 80 | |
| �ܽ��/g | Na2CO3 | 7.00 | 12.5 | 21.5 | 39.7 | 49.0 | 46.0 | 43.9 |
| NaCl | 35.7 | 35.8 | 36.0 | 36.3 | 36.6 | 37.3 | 38.4 | |
��̼���ƺ��Ȼ��Ʋ�ͬ�¶��µ��ܽ�ȣ���ش�
��1��10��ʱ��̼���Ʊ��Ȼ��Ƶ��ܽ��
��2������ͼ�����ݣ������̼��ԭ����
��3��80��ʱ����100gˮ���Ƴ�̼���Ƶı�����Һ�����������������ǣ���ȷ������0.1%��
 ��Һ�������Ϳ����о��й㷺����;�������ǵ������ܲ��ɷ֣�
��Һ�������Ϳ����о��й㷺����;�������ǵ������ܲ��ɷ֣� С��ͬѧ��������ͼ��ʾA��B���ֹ������ʵ��ܽ�����ߣ�
С��ͬѧ��������ͼ��ʾA��B���ֹ������ʵ��ܽ�����ߣ�