��Ŀ����
ʵ������һƿ������ķ�Һ��������·����ⶨ�÷�Һ�����������������ȡ����Ϊ18.2g�ĽྻС�ձ��������е���һ���������Һ�������������Ϊ33.2g��Ȼ��һö����Ϊ10.8g������������ɰֽ��ĥȥ�����⣬�����������Һ�е��������ʷ�Ӧ�������С�ձ��г�ַ�Ӧ�����������治�������ݲ���������������Ϊ43.9g���Լ���ԭ�����Һ�����������������������������С�����һλ��
��1�� �⣺���������غ㶨�ɿ�֪����Ӧ���ձ������������Ϊ����H2��������
����������Ӧ����H2��������33.2g��10.8g��43.9g��0.1g
����ȡ��Һ�����������Ϊx
H2SO4��Fe��FeSO4��H2��
98 2
x 0.1g
98 ��x��2 ��0.1
x��98��0.1g/2��4.9g ����x��4.9g��
�����⣬��ȡ��Һ������=33.2g��18.2g��15g
���� ��Һ���������������Ϊ�� 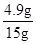 ��100%��32.7%
��100%��32.7%
��ԭ��Һ���������������Ϊ32.7% .
�����������������ϡ���ᷴӦ�������������������������ϡ���ᷴӦ������������ˮ�����������������������������������ϻ�ѧ����ʽ������������Һ��������������ٽ��������������Һ������.
���㣺���ݻ�ѧ��Ӧ����ʽ�ļ��㣻�й��������������ļ���

Ϊ�ⶨ���������Ȼ��Ƶ�̼���ƹ�����̼���Ƶ�������������ʢ��12g�û������ձ��м������ϡ������̼������ȫ��Ӧ��
��Ӧ�����þ�����������ձ���ҩƷ�������뷴Ӧʱ������ݼ�¼���£�
| ��Ӧʱ�� | t0 | t1 | t2 | t3 | t4 | t5 |
| �ձ���ҩƷ����/g | 210.0 | 206.7 | 205.9 | 205.6 | 205.6 | 205.6 |
��1������ȫ��Ӧ�����ɶ�����̼��������
��2�������������̼���Ƶ�������������ȷ��0.1%����
��3���������ȷ��ͨ������õ���̼���Ƶ�����������ʵ����ֵƫС����ԭ���� ��

 Ti + 2MgCl2������380kg���Ȼ��ѣ�����������Ϊ95%�Ľ����Ѷ���ǧ�ˣ�
Ti + 2MgCl2������380kg���Ȼ��ѣ�����������Ϊ95%�Ľ����Ѷ���ǧ�ˣ�