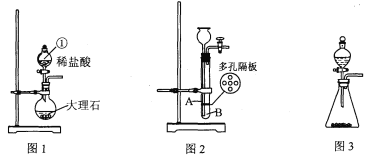
��Ŀ����
����Ŀ����������ʳ������Ӫ�����������꽡���ɳ�����Ҫ��֤��
���������ǹ���ϸ���Ļ������ʣ��ǻ�����������������֯����Ҫԭ�ϡ��������������ڵ�������ÿ����Ҫ�϶�ĵ����ʡ����������и��������ʵ��� ������ĸ����
 �ڡ�ʳƷ��ȫ����һ������Ļ��⡣����Ϊ������ʶ����ѧ���� (����ĸ)��
�ڡ�ʳƷ��ȫ����һ������Ļ��⡣����Ϊ������ʶ����ѧ���� (����ĸ)��
A.�κ�ʳƷ��������ʹ��ʳƷ���Ӽ�
B.ù��Ĵ��ס���������ʹ��������Ҳ����ʳ��
C.���ơ�п���̷������ڲ�����������ij�������Ԫ��
D.���������ƴ���ʳ��������ʳƷ
��2�����Ƽ����¡����Ϸ�չ������شٽ����������Ľ�����
�١�����������ĸ�ķ��۾�����Ŀ�����������캽ĸ����Ҫ���ϣ�����ˮ������е� �ȷ�����Ӧ����ʴ�������о�һ�ַ�ֹ������ʴ�ķ��� ��
���ɽ������ϵ��������ȷ���������ּ���������ɫ��Ⱦ������������ ���ϣ����������������ϳ�������
���𰸡��Ţ�C ��A D�Ƣ�������O2 ˢ����ָ���� ���ϳ�
��������
�����������1�����������ǹ���ϸ���Ļ������ʣ��ǻ�����������������֯����Ҫԭ�ϡ��������������ڵ�������ÿ����Ҫ�϶�ĵ����ʡ����������и��������ʵ���C������ͼ�����������ͷ�и������࣬���ͺͻ����и�����֬���߲˺�ˮ���и���ά���أ�����ʳƷ��ȫ����һ������Ļ��⡣����Ϊ������ʶ����ѧ����A�κ�ʳƷ��������ʹ��ʳƷ���Ӽ�����2�����������š���ĸ�ķ��۾�����Ŀ�����������캽ĸ����Ҫ���ϣ�����ˮ������е������ȷ����ķ�Ӧ����ʴ����ֹ������Ĵ�ʩΪˢ����ָ���ȣ����ɽ������ϵ��������ȷ���������ּ����ˡ���ɫ��Ⱦ�����������ںϳɲ���

 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�����Ŀ��ij��ȤС��ͬѧ��ʵ�����Ʊ�������������������̽��ʵ�飮
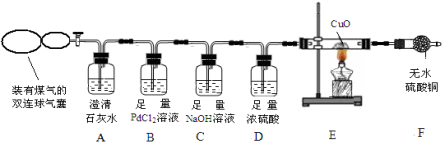
��������⡿������ͭ�Ƿ�Ҳ��������طֽ�Ĵ��������Ƿ�ȶ������̴�Ч�����ã�
����Ʋ����ʵ�顿
������3.0g����ط����Թ��м���
������3.0g�������1.0g�������̾��Ȼ�ϼ���
������x g�������1.0g�Ȼ�ͭ���Ȼ�ϼ���
��ʵ�����������
����x��ֵӦΪ ����ʵ���������ȽϿ�֤�� ����ʵ������Ӧ��Ĺ����ˮ�ܽ⡢���ˡ�ϴ�ӡ�����������õ�1.0g��ɫ��ĩ���ٽ���ɫ��ĩ��xg����ػ�ϼ��ȣ�������ʵ������ͬ���˲�����Ϊ��֤������ͭ�ڸû�ѧ��Ӧǰ�� �� �����䣮
�����ۡ�����ͭ����������طֽ�Ĵ�������д��ʵ�����еĻ�ѧ��Ӧ���ֱ���ʽ
��ʵ�鷴˼��ʵ���������Ա���Ϊ��֤��
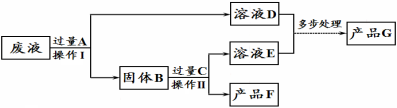
��������⡿��˫��ˮ�ֽ��ٶȻ���ʲô�����й�
����Ʋ����ʵ�顿
˫��ˮ������ | ˫��ˮ��Ũ�� | MnO2������ | ��ͬʱ���ڲ�������O2��� | |
�� | 50.0g | 1% | 0.1g | 9mL |
�� | 50.0g | 2% | 0.1g | 16mL |
�� | 50.0g | 4% | 0.1g | 31mL |
��ʵ���У�����O2�����װ���� �����ţ�
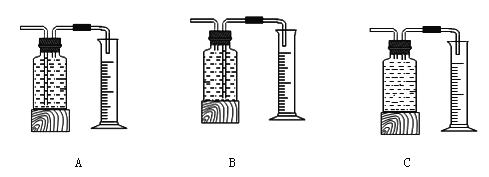
�����ۡ�����ͬ�����£�˫��ˮ��Ũ��Խ��˫��ˮ�ֽ��Խ ��