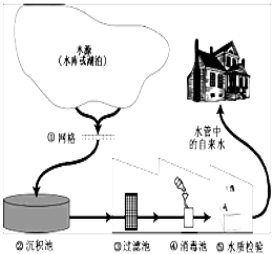
��Ŀ����
����Ŀ��ij��ѧ��ȤС���ͬѧ��ʵ����������������Ϊ20����̼������Һ��������й�ʵ�顣
��1������100g��������Ϊ20����̼������Һ��
�����Ʒ���A����Ҫ̼���ƹ��������Ϊ g��ˮ�����Ϊ mL(ˮ���ܶȽ��ƿ���1g��cm3)��
���Ʒ���B������40%��̼������Һϡ�ͳ�100��20%̼������Һ������ȡ40%��̼������Һ�������������ܶ�Ϊ1.4��/������ (���)��
������������������һ����̼������Һ�������²���˳����С�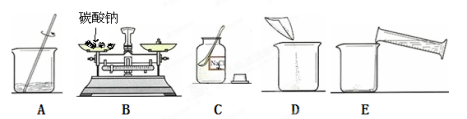
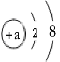
������ͼ��ʾ�������ʾ��ȷ���Ƹ���Һ�IJ���˳��Ϊ ��
����������ƽ���������̼����ʱ������������ƽ��ָ��ƫ�����̣�Ӧ ��
A�������������� B��������������
C���������� D����������
������������ԭ����ܵ����䵽��̼������Һ������������С��8%���ǣ� ��
A�������������
B����ˮʱ���Ӷ���
C����ú�װ���Լ�ƿ��ʱ������Һ������
D����ˮʱ���Ӷ���
��2��ȡ�������Ƶõ���20%��̼������Һ53�ˣ�����68.4��ijŨ�ȵ�ϡ�����ǡ����ȫ��Ӧ����Ӧ��������Һ����������������
���𰸡���1����20�� 80�� 35.7����CBDEA����B����AB ����2��10%��
��������
�����������1�������Ʒ���A����Ҫ̼���ƹ��������Ϊ100g��20%=20g��ˮ�����Ϊ100-20=80mL�����Ʒ���B������Ҫ40%��̼������ҺΪxml
X��40%��1.4g/mi=100��20% X=35.7ml������������������һ����̼������Һ�������²���˳���������ͼ��ʾ�������ʾ��ȷ���Ƹ���Һ�IJ���˳��ΪCBDEA������������ƽ���������̼����ʱ������������ƽ��ָ��ƫ�����̣�Ӧ���ٹ��壻������������ԭ����ܵ����䵽��̼������Һ������������С��8%����A�������л������ʣ�B����ˮʱ���Ӷ�������2��ȡ�������Ƶõ���20%��̼������Һ53�ˣ�����68.4��ijŨ�ȵ�ϡ�����ǡ����ȫ��Ӧ����Ӧ��������Һ��������������
�跴Ӧ��������Һ����������ΪX,���ɶ�����̼������ΪY
Na2CO3+2HCl=2NaCl+H2O+CO2��
106 117 44
20%��53g X y
106��117=20%��53g:X 106��44=20%��53g:y
X=11.7g y=4.4g
��Ӧ����Һ������Ϊ��53g+63.4g-4.4g=112g
��Ӧ����Һ��������������Ϊ��![]()

����Ŀ����8�֣����ꡰ����������ȼ�ա�ʵ���ij��ȤС����һЩ�ɻ�����⣬�������ǽ���������̽���������һͬ���롣
[����1]ϸ��˿��������ȼ��Ϊʲô�ᡰ�������䡱?
[��������1]���ճ������еĸ�����Ʒ����������̼���ʡ� ����̼ϸ��˿ȼ��ʱ�����е�̿�����ɵ�CO2����������Һ̬�������γ����ݣ�����Һ̬����������ը�����𡰻������䡱������
[���ʵ��]Ϊ��̽��ϸ��˿�ڴ�����ȼ�ղ������������䡱�����ԭ��С��ͬѧ��Ƶ�ʵ�鷽���ǣ�ȡ���̴�ϸ��ͬ�ĺ�̼ϸ��˿�Ͳ���̼��ϸ��˿���ֱ���������������ȼ�գ��۲���������Ϊ���ǵķ��� ���������������������
[ʵ��̽��1]С����ϸ��˿�������е�ȼ��ʵ��ʱ������ϸ��˿�Ƴ�����״��һ��ϵ��һ����˿�ϣ���һ��ϵ��һ�����ȼ����Ѹ�ٰ���˿��ͬ���һ����뼯��ƿ�²���û�нӴ���ϸɳ����ͼ��ʾ����
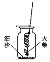
��������˿ȼ�գ�����ʵ���δ�ɹ���
��ͬѧ��Ϊ���ܵ���ʧ�ܵ�ԭ����Ҫ�����֣�
A����˿�������⼣��Ӱ���˷�Ӧ�Ľ��У�
B����ȼ�����������������ƿ�ڣ����ȼ����������������������˿��ȼ�գ�
C�����ȼ��ʱ�д����������ų�������˿�ͻ��Ѹ����������ƿ�²�������������֮�ʡ�ƿ�������������ݳ���ʹ��˿��ȼ�ա�
ʵ�飺�������ϼ��裬����ʵ��Ӧ������Ӧ�Ľ�Ϊ��
��1�� �� ��2�� ����3�� ��
[����2]��ȼ�յIJ���Ϊʲô����Fe2O3�أ�
[��������2]��Fe3O4��Fe2O3�ķֽ��¶ȡ������۵��������Fe2O3����ʱ�ֽ��Fe3O4
[����̽��]����ʵ��������ϱ������ݣ�����֪����������ȼ��ʱ�����ĸ���Ӧ��_____֮�䣬�ڴ��¶ȷ�Χ��Fe2O3�ѷֽ⣬��������������ȼ�յIJ�����Fe3O4��
Fe3O4 | Fe2O3 | �� | |
�ֽ��¶�/�� | 1538 | 1400 | �� |
�۵�/�� | �� | �� | 1535 |
[��չ����]
С��ͬѧ���֡���˿���ڴ���������ȼ���������۲쵽�������䣬���к�ɫ�������ɣ���д���÷�Ӧ�����ֱ���ʽ ��
��2����Щ���е�ʳƷ�ܷ��װ�е������ڷ��к�ɫ��FeO��ĩ������ĩ����ɫ_____����˵����װ����������������ۻ�Ա���ܼ�ʱ���ֲ�������