��Ŀ����
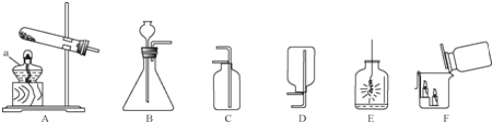
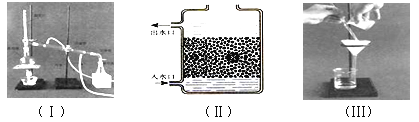
����Ŀ����7�֣�ˮ��Ӳ�Ȼ������¶ȵĸı���ı䡣����һ��ij��ѧ��ȤС���������еĻ�ѧ֪ʶ������ͼ��ʾװ�ð�����ѧʵ������ȡ������ˮ��
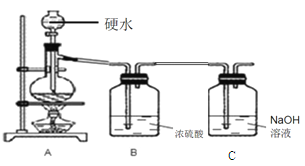
ȡʵ����ֻ��Ca(HCO3)2��Ӳˮ500.00ml���ܶ�Ϊa g/mL������ʵ�飨ʵ���¶Ȳ�����100������A�з����Ļ�ѧ��Ӧ����ʽ��Ca(HCO3)2![]() CaCO3��+H2O+CO2��
CaCO3��+H2O+CO2��
ʵ��ǰ���C������Ϊ100.00g����Ӧ��ȫ����C������Ϊ100.44g��
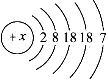
��1��Ũ����������� ��
��2������CO2 ����Ϊ g��
��3��ʵ���������ƿ���ж���ˮ��(����)���ɣ�д��������̣���
��4�������Ͽ��Ƶ� g��ˮ��ԭˮ��Ӳ��Ϊ �ȣ���ʾ��ÿһ�ȼ��൱��ÿ��ˮ�к�10.00mgCaO����
���𰸡���1������ˮ���� ��2��0.44 ��3��1g ��4��500a-1.26 112
��������
���������(1)Ũ���������ˮ�ԣ����������������Ũ�����������������ˮ����
(2)װ��C�е�NaOH��Һʱ���ն�����̼���壬������Ӧ��2NaOH + CO2 == Na2CO3 + H2O ������ʵ��ǰ���C������Ϊ100.00g����Ӧ��ȫ����C������Ϊ100.44g���ʸ��������غ㶨�ɣ�����CO2 ����=100.44g -100.00g =0.44 g
(3)���ݻ�ѧ��Ӧ��Ca(HCO3)2��CaCO3��+H2O+CO2����CaCO3��CO2��������ϵ���������̼��Ƶ�����
�⣺��̼�������Ϊx
Ca(HCO3)2��CaCO3��+H2O+CO2��
100 44
x 0.44 g
100��44=x��0.44 g
X=1 g
(4)���ݣ�3���ļ���������̼���1 g��������̼����0.44 g��ͬʱ����ˮΪ0.18g���������Ͽ��Ƶ���ˮ������=500a-1-0.44 g+0.18g=500a-1.26�����������غ㶨�ɣ�Ԫ�ص��������䣬 ��500.00mlӲˮ�й�1g̼����൱�������Ƶ�����=1g��40%��40/56��100%=0.56g=560mg����ô��ÿ��ˮ�к�������560mg��2=1120 mg����ԭˮ��Ӳ��=1120mg/10.00mg=112

 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�