��Ŀ����
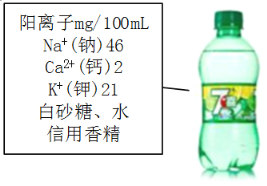
����Ŀ���γ�ʢ�����Ρ�ij��ѧ��ȤС��ͬѧ���г��������Σ�����ʵ���ҽ����ᴿ��
�����ܽ⣬���ˣ������Ȳ����У����õ��IJ���������______(����������)��
������50g�����֔�Ϊ6%��NaC1��Һ�������NaCl����_____g�����ô�������������Һ����������Һ��NaCl������������_____(�ƫ��ƫС������Ӱ�조)��
̽���:��NaClΪԭ�Ͽ�����ȡ�����ơ���ȤС��ͬѧ��ΪNa�ǻ��ý���������CuSO4������Һ��Ӧ��������Ӧ�Ľ������ʡ�
��������⣩Na��CuSO4��Һ��Ӧ���Ƿ���Cu���ɣ�
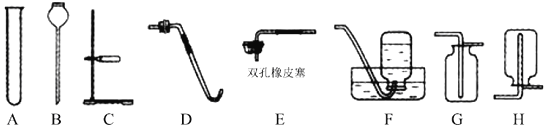
�����ʵ�飩��ȤС��ͬѧ����ʦ��ָ���£������ͼװ�ý���ʵ�顣

I.����Թ��м���һ�����ú�͡�
II.��С����һС���ƣ�������Թܣ�������Ƥ����
III.������ͨ������©�����Թ��м���CuSO4��Һ��ʹú�͵�Һ�������������رջ������۲�����
��ʵ������
(1)Na��ú������Һ���紦��������������������ݣ���Сֱ����ʧ��
(2)��Һ��ɫ�䵭������ɫ��״������������ɫ�������ɡ�
(3)��������ȼ�ŵ�ľ�����ڼ���ܿڴ������屻��ȼ��
���������ϣ���Naͨ�������ú���У���ú�Ͳ�����ˮ�Ҳ���ˮ��Ӧ��
��2Na+2H2O=2NaOH+H2������Cu(OH)2 ��CuO+H2O��
��ʵ�������
�ٸ���������Ϣ���ɻ�֪�����Ƶ�����������_____(���һ�㼴��)��
(2)ʵ���г���©��������________(���������)��
�ٱ�������CuSO4��Һ ���ռ����� �۷�ֹѹǿ����忪����
(3)���ڲ��������壬��Щͬѧ������H2����Щͬѧ������SO2��С��ͬѧ�������ۣ��ų���SO2��������________��������һ��ʵ�飬ȷ��������H2.
(4)����Ӧ��Ļ������ˣ��������μ�����ϡ���ᣬ������ȫ�ܽ⡣д��������ϡ���ᷴ�ɵĻ�ѧ����ʽ________(д��һ������)��
��ʵ����ۣ�Na��CuSO4��Һ��Ӧ����Cu���ɡ�
��ʵ�鷴˼��Na��CuSO4��Һ��Cu���ɣ�ԭ�������_______��
��������������ȡһ��������CuSO4��Һ���������ʵ�顣�������ݣ�������ɫ��Һ��������������___________��(д��������̣�����ķ�̪��Һ�������Բ��ơ�)

���𰸡� ������ 3 ƫС Ӳ��С���ܶȱ�ˮС����ú�ʹ� �٢� SO2����ȼ Cu(OH)2+H2SO4==CuSO4+2H2O����CuO+H2SO4==CuSO4+2H2O�� �ƵĻ�ѧ���ʷdz����ã�����������ͭ��Һ�е�ˮ��Ӧ������NaOH 2.84%
�������������ܽ⣬���ˣ������Ȳ����У����õ��IJ��������Dz���������50g��������Ϊ6%��NaC1��Һ���Ȼ��Ƶ�����Ϊ50g![]() 6%=3g�����ô�������������Һ����������Һ��NaCl������������ƫС��
6%=3g�����ô�������������Һ����������Һ��NaCl������������ƫС��
��ʵ�������
�ٸ��ݽ���������С���и˵��������Ӳ��С����������ú�ͺ���Һ���紦������������֪���ܶȱ�ˮС����ú�ʹ�
(2)ʵ���г���©���������Ǣٱ�������CuSO4��Һ,�۷�ֹѹǿ����忪��������ѡ�٢�;
����������ȼ�գ��ʲ������Ƕ���������������������������ͭ������ͭ���ʷ�Ӧ����ʽΪCu(OH)2+H2SO4==CuSO4+2H2O����CuO+H2SO4==CuSO4+H2O����Na��CuSO4��Һ��Cu���ɣ�ԭ��������ƵĻ�ѧ���ʷdz����ã�����������ͭ��Һ�е�ˮ��Ӧ������NaOH���ʲ���������ͭ�����û���Ӧ��
������������
���������������Ʒ�Ӧ���������Ƶ�����Ϊx
2 NaOH+ H2SO4==== Na2SO4+ 2H2O
98 142
10g![]() x
x
![]() =
=![]() �����x=1.42g
�����x=1.42g
��������6.4g��ͭ����6.4g��ͭ��������ͭ������Ϊ6.4g![]() =0.98g��������0.98g��������ͭ��ͬʱ���������Ƶ�����Ϊy
=0.98g��������0.98g��������ͭ��ͬʱ���������Ƶ�����Ϊy
2 NaOH+ CuSO4= Na2SO4+Cu��OH��2![]()
142 98
y 0.98g
![]() =
=![]() �����y=1.42g��
�����y=1.42g��
��100g ��Һ�������Ƶ�����Ϊ1.42g+1.42g=2.84g
��ɫ��Һ��������������Ϊ2.84g![]() =2.84%
=2.84%

 ��������ܸ�ϰϵ�д�
��������ܸ�ϰϵ�д�