��Ŀ����
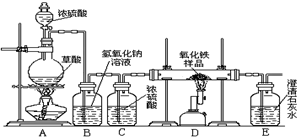 ��ѧС���ͬѧ���о���ѧϰ����չʾ��һ����ͼ��ʾ��ʵ��װ�ã�����ÿ����ѧ��Ӧ����ȫ����������Ʒ�е����ʲ��μӷ�Ӧ���������в����������������ϣ�������Ũ�������ʱ���ȷ������·�Ӧ��
��ѧС���ͬѧ���о���ѧϰ����չʾ��һ����ͼ��ʾ��ʵ��װ�ã�����ÿ����ѧ��Ӧ����ȫ����������Ʒ�е����ʲ��μӷ�Ӧ���������в����������������ϣ�������Ũ�������ʱ���ȷ������·�Ӧ��H2C2O4
| ||
| �� |
ͨ�����ۣ�ͬѧ�Ƕ�����װ�����˶�����ʶ��
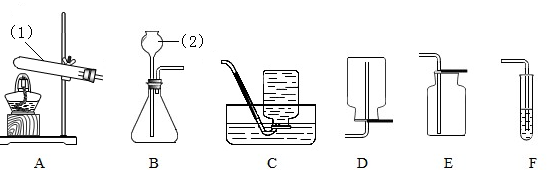
��1����һС��ͬѧ˵����ʵ�鰲ȫ��ʵ�������������Ҫ���װ�õ������ԣ�ʵ�鿪ʼ�ȼ���
A
A
����A��D������ʵ�����ʱ��Ӧ��
��
�����Ȼ��ֹͣD���ļ��ȣ��ӻ����ĽǶȽ�����Eװ�ú�Ӧ��β�����д�����Ҫ����β����ԭ���ǣ���2���ڶ�С���ͬѧ˵���ø�ʵ��װ�ÿ��Լ���һ����̼����������Ӧ�IJ������Bװ�õ�����
����A�з�Ӧ���ɵĶ�����̼
����A�з�Ӧ���ɵĶ�����̼
��Eװ�õ��������鲢����D�з�Ӧ���ɵĶ�����̼
���鲢����D�з�Ӧ���ɵĶ�����̼
��CO��Fe2O3��Ӧ�Ļ�ѧ����ʽΪ3CO+Fe2O3
3CO2+2Fe
| ||
3CO+Fe2O3
3CO2+2Fe
���÷�Ӧ
| ||
��
��
�����ǡ����û���Ӧ����֤��D������������2�������ǣ�����
��3������С���ͬѧ˵����������װ�û����Բⶨ��������Ʒ�����������������������ǵIJⶨ��
���ǣ�������������Ʒ������10.0g����Ʒ�벣���ܵ�������Ϊ60.0g����ȫ��Ӧ����ȴ���ٳ�����������ʣ������������Ϊ57.6g����ʵ������������Ʒ������������������Ϊ
80%
80%
%����4������С��ͬѧ˵����������װ�û�������ⶨ��Ʒ�������������������ķ������ȳ�����������Ʒ���������ٷֱ����Eװ���ڷ�Ӧǰ��������������ɼ��������Ʒ�������������������������˷���ʵ��ʵ��ⶨ���ȴƫ�������ƫ���ԭ�������
Bװ������CO2����֣���E������װ��������е�CO2�������CO2����ĸ��ţ�
Bװ������CO2����֣���E������װ��������е�CO2�������CO2����ĸ��ţ�
����5����˼��Ҫ��֤����ֽ���CO2���ɣ�������װ��A��B֮������һ������B��ʢ
����ʯ��ˮ
����ʯ��ˮ
��ϴ��ƿ�����û��װ��C����������������������������
����
����ƫ��ƫС�����䣩����������������ԭ��һ����̼��ԭ��������ʵ���������������ȡ������һ����̼��ʵ�飬��������Ϣ������ֽ��õ���һ����̼�л��ж�����̼��ˮ���������ҪԤ�ȳ���������̼�����������������̼��Ӧ���Ķ�����̼��Ũ���������ˮ�Գ�ȥˮ�֣������Ķ�����̼ʹ����ʯ��ˮ����ǣ������п���һ����̼���࣬�����β�����д�������3������������ԭΪ�����������ᣬ���ò����������������������������������������ʽ���㣮
����⣺��1��CO�ǿ�ȼ�����壬������ȼ�ᷢ����ը������Ӧ����CO��������װ�ã���ʵ�鿪ʼʱ�ȼ���A���ľƾ��ƣ�ʹ������һ����̼�ž�װ���ڵĿ�����ʵ�����ʱ��Ϊ��ֹ����������������Ҫ����ͨһ��COֱ��װ����ȴ������Ҫ��Ϩ��D���ƾ��ƣ��ӻ����ĽǶȿ���Ϊ��ֹ�ж���δ��Ӧ��һ����̼ͨ��E���������Ӧ��E���һ����̼���д������ɲ�ȡ�������ռ����ȼ�ķ�����
��2��Bװ�������������������ղ����Ķ�����̼��Eװ���������ղ����������̼�Ĵ��ڣ�Dװ���з�Ӧ�Ļ�ѧ����ʽΪ��3CO+Fe2O3
3CO2+2Fe��
�û���Ӧ�ǵ����뻯���ﷴӦ��������ĵ��ʺͻ�����Ļ�ѧ��Ӧ������Ӧ����û�е��ʣ������������û���Ӧ��
��3��ԭ����ƷΪ��������10g���������ܵ�����Ϊ50g���ʷ�Ӧ��Ϊʣ�����Ϊ��7.6g�������ٵ�����Ϊ2.4g��ʵ�ʾ�����Ԫ�ص����������ò���������
��������������ΪX
3CO+Fe2O3
3CO2+2Fe��m
160 112 48
x ��60.0-57.6��g
=
��
��ã�x=8g
������������������
��100%=80%
��4��Eװ���ڷ�Ӧ�����ӵ������Ƿ�Ӧ�����ɵĶ�����̼�����������Bװ������CO2����ֻ�E������װ��������е�CO2�������CO2����ĸ��ţ��ͻ�ʹ������̼����������ʵ�ʲⶨ����ͻ�ƫ��
��5��������̼��ʹ����ʯ��ˮ����ǣ�������װ��A֮ǰ����װ��A��B֮������һ������B��ʢ����ʯ��ˮ��ϴ��ƿ��
���û��Cװ�ã���û������CO�е�ˮ�֣������ǰ���Dװ�������ݽ��м��㣬�Ƿ�����ˮ�ֶԲⶨ���û��Ӱ�죮
�ʴ�Ϊ����1��A���ȣ���ֹ�ж�����һ����̼��Ⱦ������
��2������A�з�Ӧ���ɵĶ�����̼������D�з�Ӧ���ɵĶ�����̼��3CO+Fe2O3
3CO2+2Fe����
��3��80%��
��4��Bװ������CO2����֣�E������װ��������е�CO2�������CO2����ĸ��ţ�����һ�������𰸼��ɣ���
��5������ʯ��ˮ�����䣮
��2��Bװ�������������������ղ����Ķ�����̼��Eװ���������ղ����������̼�Ĵ��ڣ�Dװ���з�Ӧ�Ļ�ѧ����ʽΪ��3CO+Fe2O3
| ||
�û���Ӧ�ǵ����뻯���ﷴӦ��������ĵ��ʺͻ�����Ļ�ѧ��Ӧ������Ӧ����û�е��ʣ������������û���Ӧ��
��3��ԭ����ƷΪ��������10g���������ܵ�����Ϊ50g���ʷ�Ӧ��Ϊʣ�����Ϊ��7.6g�������ٵ�����Ϊ2.4g��ʵ�ʾ�����Ԫ�ص����������ò���������
��������������ΪX
3CO+Fe2O3
| ||
160 112 48
x ��60.0-57.6��g
| 160 |
| x |
| 48 |
| 2.4g |
��ã�x=8g
������������������
| 8g |
| 10g |
��4��Eװ���ڷ�Ӧ�����ӵ������Ƿ�Ӧ�����ɵĶ�����̼�����������Bװ������CO2����ֻ�E������װ��������е�CO2�������CO2����ĸ��ţ��ͻ�ʹ������̼����������ʵ�ʲⶨ����ͻ�ƫ��
��5��������̼��ʹ����ʯ��ˮ����ǣ�������װ��A֮ǰ����װ��A��B֮������һ������B��ʢ����ʯ��ˮ��ϴ��ƿ��
���û��Cװ�ã���û������CO�е�ˮ�֣������ǰ���Dװ�������ݽ��м��㣬�Ƿ�����ˮ�ֶԲⶨ���û��Ӱ�죮
�ʴ�Ϊ����1��A���ȣ���ֹ�ж�����һ����̼��Ⱦ������
��2������A�з�Ӧ���ɵĶ�����̼������D�з�Ӧ���ɵĶ�����̼��3CO+Fe2O3
| ||
��3��80%��
��4��Bװ������CO2����֣�E������װ��������е�CO2�������CO2����ĸ��ţ�����һ�������𰸼��ɣ���
��5������ʯ��ˮ�����䣮
���������⿼����һ����̼��ԭ�������ķ�Ӧԭ�������衢ʵ������β�������Լ�Ӧ�ã�Ҫע���ж����岻�������ŷţ����뾭�������������������ŷţ�

��ϰ��ϵ�д�
 �����ҵ���������ϵ�д�
�����ҵ���������ϵ�д� �����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д�
�����̸�Ӯ����ٸ�Ч�����ܸ�ϰ���ϿƼ�������ϵ�д� �����ҵ�����������ѧ���ӳ�����ϵ�д�
�����ҵ�����������ѧ���ӳ�����ϵ�д�
�����Ŀ





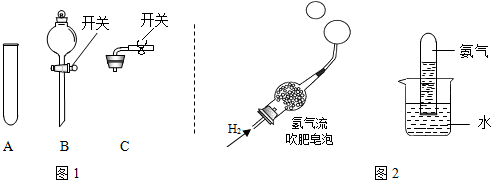
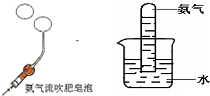 ��ѧ��ȤС���ͬѧ���о�����������ʱ��������ͼ����ʵ�飺
��ѧ��ȤС���ͬѧ���о�����������ʱ��������ͼ����ʵ�飺