��Ŀ����
��ѧ��٤����˵����һ������������ӹ۲���ʵ���е���������������ʦ�ṩ��һЩʵ��װ�ã������Ҫ��ش��������⣺
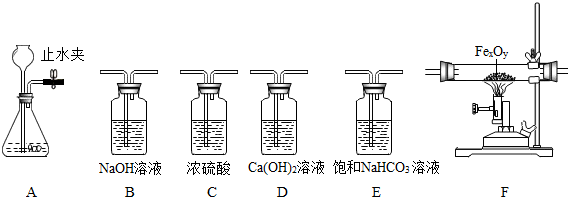
��1���ô���ʯ��ϡ���ᷴӦ��ϡ����ӷ�����HCl������ñ���NaHCO3��Һ���գ���ȡ���ռ����﴿���Ķ�����̼���壮
�ٹر� Aװ���е�ֹˮ�кӳ���©������ƿ��ע��һ������ˮ����ֹ����ͼ��ʾ����Aװ���Ƿ�©����______���©����������©��������ȷ����֮һ����
��ʵ������ȡ������̼�Ļ�ѧ����ʽ��______��
����ѡ����������˳��______�������ռ�װ�ã���д���������ĸ����
��2��ijͬѧ���ú��ж�����̼��ˮ�������ʵ� CO�ⶨһ�����������FexOy������ɣ����������������������Ϊ 23.2g�����ʵ�鷽�������������Ǵ������ң�ѡ��������� B��C��F��D ˳�����ӣ�Ȼ�����ʵ�飨�����йط�Ӧ����Ӧ��ȫ������
��װ��B��������______��
��Fװ���е�FexOyȫ������ԭ����ʣ����������Ϊ16.8g��������������Ļ�ѧʽΪ______��
����������������ʱ�����û��Bװ�ã��ⶨ�������Ԫ������Ԫ�ص�������ֵ��______ ���ƫ��ƫС�����������䡱֮һ����
�ܸ���װ������һ�����ԵIJ���֮����Ӧ��װ��D�����ȼ�ŵľƾ��ƴ���β������������Ŀ����______��
�⣺��1������װ��©����װ���ڵ����������ݳ���װ���ڵ�����ѹǿ��С����������ѹ�������³���©���ڵ�Һ�潫���½���������װ���ڵ�Һ��û���½���˵��װ�ò�©����
��ʵ������ȡCO2�����ҩƷʱ����ʯ��ϡ���ᣬ��Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼���÷�Ӧ��ѧ����ʽ��CaCO3+2HCl=CaCl2+H2O+CO2����
�۷���װ���ǹ�Һ��Ӧ��Aװ�ã���ˮ��Ũ���ᣬ��ȥ�Ȼ����ñ���̼��������Һ������ȳ���ˮ����ͨ��̼��������Һʱ���ִ���ˮ��ǰ���ˮ�Ͱ׳��ˣ����ѳ�ˮ����������Ҫ��ͨ������̼��������Һ��ȥ�Ȼ��⣬��ͨ��Ũ�����ˮ������ռ���������˳��Ϊ��A��E��C��
��2��������Bװ��װNaOHĿ���ǽ�һ����̼��������������̼���еĶ�����̼��ȥ������Ӱ��ʵ������
����ȫ��Ӧ��װ��C��ʣ���������16.8g����������������������������װ��C���ٵ�����Ϊ����������Ԫ�ص�������23.2g-16.8g=6.4g��
��������������ﻯѧʽΪFexOy������������Ԫ�غ���Ԫ�ص�������Ϊ��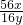 =
=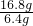 ��������
��������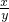 =
=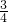 �����Ը�����������Ļ�ѧʽ��Fe3O4��
�����Ը�����������Ļ�ѧʽ��Fe3O4��
��װ��B�dz�ȥ��������еĶ�����̼����Cװ�õ������仯����Ӱ�죬���ԶԲⶨ����������Ļ�ѧʽ�����Ӱ�죻
����Ϊһ����̼�ж�������Ⱦ���������װ��D�����ȼ�ŵľƾ��ƴ���β����Ŀ���ǣ���ֹһ����̼��Ⱦ������
�ʴ�Ϊ����1����©����CaCO3+2HCl=CaCl2+H2O+CO2����A��E��C��
��2����ȥ������̼��Fe3O4���������䣻��ֹһ����̼��Ⱦ������
��������1���ٴ�ͼ�п��Կ������ܱյ������У����Ը���ѹǿ���жϷ�����Һ��û���½���
�ڸ���ʵ������ȡCO2�����ҩƷʱ����ʯ��ϡ���ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼��д����Ӧ�ķ���ʽ��
�۸��ݶ�����̼�����ʿ���֪�����������Ũ���ᣬ�������Ȼ�����������ñ��͵�̼������Һ�����Ծݴ��������⣻
��2���ٸ��������װ�ý��з�������װ����Ƶ�Ŀ�Ľ��н��
�ڸ��ݶ�����װ�������ķ�����Cװ�ü��ٵ�������������������Ԫ�ص�������������������������������Ԫ������Ԫ�ص�������������������������Ԫ�غ���Ԫ�ص����������������Ļ�ѧʽ��
�۷���Ϊ�ⶨ�������������ɸ�װ�õ����ã��Ը�װ�ý������ۣ����ж϶Բⶨ�����������Ӱ�죻
����ΪCO���ж��ԣ�������ʵ����Ҫ��β��CO��������������
��������1�����ʵ������ȡ������̼�ķ�Ӧԭ����ʵ�鲽�衢װ��ʾ��ͼ��ע�������Լ����ʵļ���ͳ��ӵ�֪ʶ���������׳����ĵط�������˳��Ҫ��dz�����ʱ����Ҫ��ѭ�������̳�����ԭ���ڳ�ˮʱҪ�ŵ�������������ͨ��������Һʱ�ֻ����ˮ��
��2��������һ��̽����ʵ���⣬���Կα���һ����̼��ԭ������Ϊ���ݣ�������֪ʶ�ı仯�ǶȺ�Ǩ�������Ŀ��飬CO�Ļ�ԭ�Կ��Խ������������е�����ԭ����������ʵ��Ŀ��ȷ����Ƶ�ʵ��װ�õ����ã�ͬʱע��һ����̼�Ķ��ԣ�Ҫ�������������������Ⱦ��
��ʵ������ȡCO2�����ҩƷʱ����ʯ��ϡ���ᣬ��Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼���÷�Ӧ��ѧ����ʽ��CaCO3+2HCl=CaCl2+H2O+CO2����
�۷���װ���ǹ�Һ��Ӧ��Aװ�ã���ˮ��Ũ���ᣬ��ȥ�Ȼ����ñ���̼��������Һ������ȳ���ˮ����ͨ��̼��������Һʱ���ִ���ˮ��ǰ���ˮ�Ͱ׳��ˣ����ѳ�ˮ����������Ҫ��ͨ������̼��������Һ��ȥ�Ȼ��⣬��ͨ��Ũ�����ˮ������ռ���������˳��Ϊ��A��E��C��
��2��������Bװ��װNaOHĿ���ǽ�һ����̼��������������̼���еĶ�����̼��ȥ������Ӱ��ʵ������
����ȫ��Ӧ��װ��C��ʣ���������16.8g����������������������������װ��C���ٵ�����Ϊ����������Ԫ�ص�������23.2g-16.8g=6.4g��
��������������ﻯѧʽΪFexOy������������Ԫ�غ���Ԫ�ص�������Ϊ��
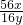 =
=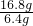 ��������
��������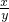 =
=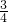 �����Ը�����������Ļ�ѧʽ��Fe3O4��
�����Ը�����������Ļ�ѧʽ��Fe3O4����װ��B�dz�ȥ��������еĶ�����̼����Cװ�õ������仯����Ӱ�죬���ԶԲⶨ����������Ļ�ѧʽ�����Ӱ�죻
����Ϊһ����̼�ж�������Ⱦ���������װ��D�����ȼ�ŵľƾ��ƴ���β����Ŀ���ǣ���ֹһ����̼��Ⱦ������
�ʴ�Ϊ����1����©����CaCO3+2HCl=CaCl2+H2O+CO2����A��E��C��
��2����ȥ������̼��Fe3O4���������䣻��ֹһ����̼��Ⱦ������
��������1���ٴ�ͼ�п��Կ������ܱյ������У����Ը���ѹǿ���жϷ�����Һ��û���½���
�ڸ���ʵ������ȡCO2�����ҩƷʱ����ʯ��ϡ���ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼��д����Ӧ�ķ���ʽ��
�۸��ݶ�����̼�����ʿ���֪�����������Ũ���ᣬ�������Ȼ�����������ñ��͵�̼������Һ�����Ծݴ��������⣻
��2���ٸ��������װ�ý��з�������װ����Ƶ�Ŀ�Ľ��н��
�ڸ��ݶ�����װ�������ķ�����Cװ�ü��ٵ�������������������Ԫ�ص�������������������������������Ԫ������Ԫ�ص�������������������������Ԫ�غ���Ԫ�ص����������������Ļ�ѧʽ��
�۷���Ϊ�ⶨ�������������ɸ�װ�õ����ã��Ը�װ�ý������ۣ����ж϶Բⶨ�����������Ӱ�죻
����ΪCO���ж��ԣ�������ʵ����Ҫ��β��CO��������������
��������1�����ʵ������ȡ������̼�ķ�Ӧԭ����ʵ�鲽�衢װ��ʾ��ͼ��ע�������Լ����ʵļ���ͳ��ӵ�֪ʶ���������׳����ĵط�������˳��Ҫ��dz�����ʱ����Ҫ��ѭ�������̳�����ԭ���ڳ�ˮʱҪ�ŵ�������������ͨ��������Һʱ�ֻ����ˮ��
��2��������һ��̽����ʵ���⣬���Կα���һ����̼��ԭ������Ϊ���ݣ�������֪ʶ�ı仯�ǶȺ�Ǩ�������Ŀ��飬CO�Ļ�ԭ�Կ��Խ������������е�����ԭ����������ʵ��Ŀ��ȷ����Ƶ�ʵ��װ�õ����ã�ͬʱע��һ����̼�Ķ��ԣ�Ҫ�������������������Ⱦ��

��ϰ��ϵ�д�
�����Ŀ


