题目内容
科学家伽利略说:一切推理都必须从观察与实验中得来”以下是老师提供的一些实验装置,请根据要求回答下列问题:

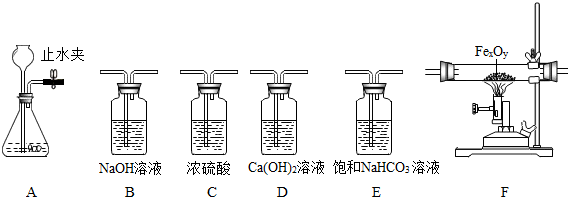
(1) 用大理石和稀盐酸反应 (稀盐酸挥发出的HCl气体可用饱和NaHCO3溶液吸收) 制取并收集干燥纯
的二氧化碳气体。
① 关闭A装置中的止水夹后,从长颈漏斗向锥形瓶中注入一定量的水,静止后如图所示, 则A装置是否漏气? (填“漏气”、“不漏气”或“无法确定”之一)。
② 实验室制取二氧化碳的化学方程式是
③ 所选仪器的连接顺序 → 气体收集装置 (填写仪器序号字母)
(2) 某同学欲用含有二氧化碳和水蒸气杂质的CO测定一种铁的氧化物 (FeXOY) 的组成,称量该铁的氧化物的质量为23.2g。设计实验方案:气体流向是从左向右,选择的仪器按B→C→F→D顺序连接,然后进行实验 (假设有关反应均反应完全)。
①装置B的作用是 。
② F装置中的FeXOY 全部被还原后,称量剩余固体的质量为16.8g,该铁的氧化物的化学式
为
③ 用上述方法测量时,如果没有B装置,测定结果中铁元素与氧元素的质量比值会 (填“偏大”“偏小”“基本不变”之一)
④ 该套装置中有一个明显的不足之处,应在装置D后放置燃着的酒精灯处理尾气,这样做的目的
是
 天天向上一本好卷系列答案
天天向上一本好卷系列答案 小学生10分钟应用题系列答案
小学生10分钟应用题系列答案