��Ŀ����
һ̼��ѧ���Է�����ֻ��һ��̼ԭ�ӵĻ������CO��CH4�ȣ�Ϊԭ�����ϳ�һϵ�л���ԭ�Ϻ�ȼ�ϵĻ�ѧ��
��1����ҵ������Ȼ����ˮ�����ڸ��������·�����Ӧ���õ�CO��H2���÷�Ӧ�Ļ�ѧ����ʽΪ
��2��ij��ѧ��ȤС���ͬѧ��ʵ����ģ���˺ϳ������Ʊ��������ʵ����֤�ϳ����Ļ�ԭ�ԣ�
I���ü����ˮ�����ڸ��������·�Ӧ�õ��ϳ��������� ��1������д�Ļ�ѧ����ʽ���ϳ�����CO��H2��������Ϊ
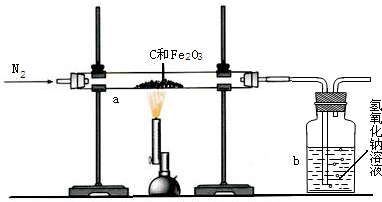
���úϳ�����ԭ��������ͭ��ʵ��װ����ͼ��ʾ��
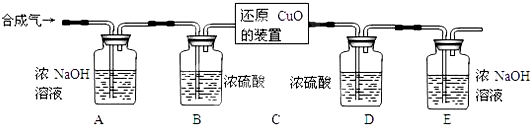
[���ʵ�鲽��]
�����Ӻ�װ�ò���������ԣ�
��װ��ҩƷ��ͨ��һ��ʱ��ϳ�����
�۷ֱ����װ��D��E��������
�ܻ���ͨ��ϳ���������װ��C��ʹ���ַ�Ӧ��
��ֹͣ���ȣ�����ͨ��ϳ������ָ������£�
���ٴηֱ����װ��D��E��������
[����ʵ�����]��������ͬѧ����Ϊ��װ��E��Ӧ����һ���ƾ��ƣ�����ʵ�鲽��
[����ʵ��]��С���ͬѧ�������������ʵ�飬����¼��ʵ����������ݣ�
��װ��C�г���
�ڳ��������������ʾ��
[������������ݴ���]
�ټ�ͬѧ����װ��C�е�������Ϊ�Ǻϳ����е�CO��H2��ԭ��CuO��
����ͬѧͨ�����ϱ����ݵķ������Ʋ����CO��H2���⣬�Ƶõĺϳ����п��ܻ���δ��Ӧ��CH4����CH4Ҳ��ԭ��CuO����ͨ������˵�������Ʋ����ݣ�
[��չ̽��]��С��ͬѧ����CH4�Ƿ�����ܹ���ԭ����ͭ��������ɲ�����̽����
�������ϣ�������л�ԭ�ԣ����Ի�ԭ����ͭ������CO2��H2O����ɫ����ˮ����ͭ��ˮ������ɫ��
ʵ����ƣ���С��ͬѧ���ô����ļ������������װ�ý���ʵ�飮
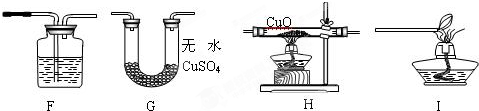
��ͼ��װ��F��ʢ�ŵ��Լ�������
��ͼ��װ�õ���ȷ����˳����
��1����ҵ������Ȼ����ˮ�����ڸ��������·�����Ӧ���õ�CO��H2���÷�Ӧ�Ļ�ѧ����ʽΪ
CH4+H2O
CO+3H2
| ||
CH4+H2O
CO+3H2
���õ���CO��H2�Ļ��������Ϊ�ϳ������ϳ����ڹ�ҵ�Ͽ����ںϳ�һϵ�л���ԭ�Ϻ�����ȼ�ϣ���������ұ��ijЩ������
| ||
��2��ij��ѧ��ȤС���ͬѧ��ʵ����ģ���˺ϳ������Ʊ��������ʵ����֤�ϳ����Ļ�ԭ�ԣ�
I���ü����ˮ�����ڸ��������·�Ӧ�õ��ϳ��������� ��1������д�Ļ�ѧ����ʽ���ϳ�����CO��H2��������Ϊ
14��3
14��3
�����úϳ�����ԭ��������ͭ��ʵ��װ����ͼ��ʾ��

[���ʵ�鲽��]
�����Ӻ�װ�ò���������ԣ�
��װ��ҩƷ��ͨ��һ��ʱ��ϳ�����
�۷ֱ����װ��D��E��������
�ܻ���ͨ��ϳ���������װ��C��ʹ���ַ�Ӧ��
��ֹͣ���ȣ�����ͨ��ϳ������ָ������£�
���ٴηֱ����װ��D��E��������
[����ʵ�����]��������ͬѧ����Ϊ��װ��E��Ӧ����һ���ƾ��ƣ�����ʵ�鲽��
��
��
����ʵ�鲽�����ţ��е�ȼ����ȼǰӦ�������鴿����[����ʵ��]��С���ͬѧ�������������ʵ�飬����¼��ʵ����������ݣ�
��װ��C�г���
��
��
ɫ���ʣ��ڳ��������������ʾ��
| װ��D������ | װ��E������ | |
| ��Ӧǰ | 292.4g | 198.2g |
| ��Ӧ�� | 296.0g | 201.5g |
�ټ�ͬѧ����װ��C�е�������Ϊ�Ǻϳ����е�CO��H2��ԭ��CuO��
����ͬѧͨ�����ϱ����ݵķ������Ʋ����CO��H2���⣬�Ƶõĺϳ����п��ܻ���δ��Ӧ��CH4����CH4Ҳ��ԭ��CuO����ͨ������˵�������Ʋ����ݣ�
��ʵ����������̼����Ԫ�ص���������һ����̼��������ԭ����ͭ��̼����Ԫ�ص������Ȳ���
��ʵ����������̼����Ԫ�ص���������һ����̼��������ԭ����ͭ��̼����Ԫ�ص������Ȳ���
[��չ̽��]��С��ͬѧ����CH4�Ƿ�����ܹ���ԭ����ͭ��������ɲ�����̽����
�������ϣ�������л�ԭ�ԣ����Ի�ԭ����ͭ������CO2��H2O����ɫ����ˮ����ͭ��ˮ������ɫ��
ʵ����ƣ���С��ͬѧ���ô����ļ������������װ�ý���ʵ�飮

��ͼ��װ��F��ʢ�ŵ��Լ�������
�����ʯ��ˮ
�����ʯ��ˮ
����ͼ��װ�õ���ȷ����˳����
HGFI
HGFI
������ĸ����ÿ��װ������һ�Σ�����������1�����ݷ�Ӧ�������ͷ�Ӧ������д����ʽ��
�����ݷ���ʽ�����ʵ���������⣻
��[����ʵ�����]�ɸ��ݷ�Ӧ����������
[����ʵ��]
�ٿɸ���������һ����̼���л�ԭ�Է������
[������������ݴ���]
�ڷ����������ݿ�֪�������ж�����̼��ˮ�����������ü�ֵ����������ȫ����CO��H2���뷴Ӧ�õ��Ķ�����̼��ˮ����������ȫ���Ǽ�����뷴Ӧ�õ��Ķ�����Ƚϣ������������Ա�̼����Ԫ�ص������ȼ����жϣ�
[��չ̽��]
��Ҫ֤��������뷴Ӧ����ͨ��֤����Ӧ������������жϣ����������̼�ó����ʯ��ˮ��
�ڸ��ݷ�Ӧ�Ĺ�����ȷ��˳����һ����֤��������֤ˮ��������Ϊ�������Һ�г������������ˮ������
�����ݷ���ʽ�����ʵ���������⣻
��[����ʵ�����]�ɸ��ݷ�Ӧ����������
[����ʵ��]
�ٿɸ���������һ����̼���л�ԭ�Է������
[������������ݴ���]
�ڷ����������ݿ�֪�������ж�����̼��ˮ�����������ü�ֵ����������ȫ����CO��H2���뷴Ӧ�õ��Ķ�����̼��ˮ����������ȫ���Ǽ�����뷴Ӧ�õ��Ķ�����Ƚϣ������������Ա�̼����Ԫ�ص������ȼ����жϣ�
[��չ̽��]
��Ҫ֤��������뷴Ӧ����ͨ��֤����Ӧ������������жϣ����������̼�ó����ʯ��ˮ��
�ڸ��ݷ�Ӧ�Ĺ�����ȷ��˳����һ����֤��������֤ˮ��������Ϊ�������Һ�г������������ˮ������
����⣺��1���������⣬��Ȼ����ˮ�����ڸ��������·�����Ӧ���õ�CO��H2�����ݷ���ʽ��дҪ��÷���ʽΪ��CH4+H2O
CO+3H2��
�ʴ�Ϊ��CH4+H2O
CO+3H2��
��2��I�����ݻ�ѧ����ʽ�����ʵ������ȿ�֪CO��H2��������=28����2��3��=14��3��
�ʴ�Ϊ��14��3��
��[����ʵ�����]װ��ҩƷ��ͨ��һ��ʱ��ϳ�����Ŀ�����ų��Թ��еĿ�������ֹ������ը���ò������岢δ���ڻ�ԭ����ͭ����һ����̼�ж���������������Һ����Ӧ�����Դ�ʱ��װ��ĩ�˵�ȼ�ƾ��ƽ���β����������ֹ��Ⱦ������
�ʴ�Ϊ���ڣ�
[����ʵ��]
��������һ����̼���л�ԭ�ԣ�������ͭ��Ӧ����ͭ������װ��C�к�ɫ������ͭ��Ϊ��ɫ��
�ʴ�Ϊ���죻
[������������ݴ���]
�ڷ�Ӧǰ��Ԫ�ص��������䣬���ֻ��һ����̼����������ԭ����CuO���ϳ�����CO��H2�ķ��Ӹ�����Ϊ1��3����������̼Ԫ������Ԫ�ص�������Ϊ12��3��2=2��1�����ֻ�м��黹ԭ����CuO����������̼Ԫ������Ԫ����������Ϊ12��4=3��1��
�ֲ�ö�����̼��������201.5g-198.2g=3.3g��ˮ��������296.0gһ292.4g=3.6g
����������̼Ԫ������Ԫ�ص������ȣ���3?3g��
��100%������3.6g��
��100% ��=9��4��
9��4����2��1��3��1֮�䣬�ɴ˿��Ʋ��������һ����̼���������⣬�Ƶõĺϳ����п��ܻ�������Ӧ�ļ��飬�Ҽ���Ҳ��ԭ��CuO��
�ʴ�Ϊ����ʵ����������̼����Ԫ�ص���������һ����̼��������ԭ����ͭ��̼����Ԫ�ص������Ȳ�����
[��չ̽��]
��Ҫ֤�����黹ԭ����ͭ��ֻҪ֤�����������ж�����̼��ˮ���ɼ��ɣ�����Fװ��Ӧ�Ǽ������ɶ�����̼�ģ��ó���ʯ��ˮ��
�ʴ�Ϊ�������ʯ��ˮ��
�ڷ�����ʵ���Ŀ�Ŀ�֪ʵ���и�װ�õ���;�ֱ��ǣ�װ��H�ǻ�ԭCuO��G������֤��ˮ�����ɣ�F��֤��������̼���ɣ�I�Ǵ���β��������˳��ΪHGFI��
�ʴ�Ϊ��HGFI
| ||
�ʴ�Ϊ��CH4+H2O
| ||
��2��I�����ݻ�ѧ����ʽ�����ʵ������ȿ�֪CO��H2��������=28����2��3��=14��3��
�ʴ�Ϊ��14��3��
��[����ʵ�����]װ��ҩƷ��ͨ��һ��ʱ��ϳ�����Ŀ�����ų��Թ��еĿ�������ֹ������ը���ò������岢δ���ڻ�ԭ����ͭ����һ����̼�ж���������������Һ����Ӧ�����Դ�ʱ��װ��ĩ�˵�ȼ�ƾ��ƽ���β����������ֹ��Ⱦ������
�ʴ�Ϊ���ڣ�
[����ʵ��]
��������һ����̼���л�ԭ�ԣ�������ͭ��Ӧ����ͭ������װ��C�к�ɫ������ͭ��Ϊ��ɫ��
�ʴ�Ϊ���죻
[������������ݴ���]
�ڷ�Ӧǰ��Ԫ�ص��������䣬���ֻ��һ����̼����������ԭ����CuO���ϳ�����CO��H2�ķ��Ӹ�����Ϊ1��3����������̼Ԫ������Ԫ�ص�������Ϊ12��3��2=2��1�����ֻ�м��黹ԭ����CuO����������̼Ԫ������Ԫ����������Ϊ12��4=3��1��
�ֲ�ö�����̼��������201.5g-198.2g=3.3g��ˮ��������296.0gһ292.4g=3.6g
����������̼Ԫ������Ԫ�ص������ȣ���3?3g��
| 12 |
| 44 |
| 2 |
| 18 |
9��4����2��1��3��1֮�䣬�ɴ˿��Ʋ��������һ����̼���������⣬�Ƶõĺϳ����п��ܻ�������Ӧ�ļ��飬�Ҽ���Ҳ��ԭ��CuO��
�ʴ�Ϊ����ʵ����������̼����Ԫ�ص���������һ����̼��������ԭ����ͭ��̼����Ԫ�ص������Ȳ�����
[��չ̽��]
��Ҫ֤�����黹ԭ����ͭ��ֻҪ֤�����������ж�����̼��ˮ���ɼ��ɣ�����Fװ��Ӧ�Ǽ������ɶ�����̼�ģ��ó���ʯ��ˮ��
�ʴ�Ϊ�������ʯ��ˮ��
�ڷ�����ʵ���Ŀ�Ŀ�֪ʵ���и�װ�õ���;�ֱ��ǣ�װ��H�ǻ�ԭCuO��G������֤��ˮ�����ɣ�F��֤��������̼���ɣ�I�Ǵ���β��������˳��ΪHGFI��
�ʴ�Ϊ��HGFI
������������һ����Ϊ�ۺϵ�ʵ��̽���⣬���Ժܺõ�����ѧ�����������������������Ҫ������Ŀ��������Ϣϸ�ķ������

��ϰ��ϵ�д�
�����Ŀ
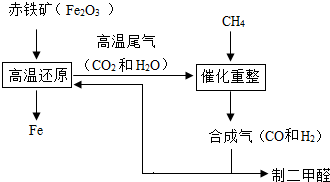 һ̼��ѧ���Է�����ֻ����һ��̼ԭ�ӵĻ������CO��CH4�ȣ�Ϊԭ�����ϳ�һϵ�л���ԭ�Ϻ�ȼ�ϵĻ�ѧ��CO�Ǵ�ú��������ϳ����õ��ģ�ú��������Ҫ��Ӧ�У�
һ̼��ѧ���Է�����ֻ����һ��̼ԭ�ӵĻ������CO��CH4�ȣ�Ϊԭ�����ϳ�һϵ�л���ԭ�Ϻ�ȼ�ϵĻ�ѧ��CO�Ǵ�ú��������ϳ����õ��ģ�ú��������Ҫ��Ӧ�У�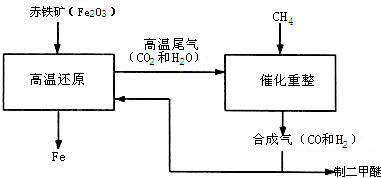
 ��2011?������һģ��һ̼��ѧ���Է�����ֻ��һ��̼ԭ�ӵĻ������CO��CH4�ȣ�Ϊԭ�����ϳ�һϵ�л���ԭ�Ϻ�ȼ�ϵĻ�ѧ����ҵ�ϳ�����Ȼ����ˮ�����������̼�����´���Ӧ�õ�CO��H2���õ���CO��H2�Ļ��������Ϊ�ϳ�������ͼΪ���úϳ����ϳ�����ȼ�϶����ѣ�CH3OCH3����ұ�����IJ����������̣�
��2011?������һģ��һ̼��ѧ���Է�����ֻ��һ��̼ԭ�ӵĻ������CO��CH4�ȣ�Ϊԭ�����ϳ�һϵ�л���ԭ�Ϻ�ȼ�ϵĻ�ѧ����ҵ�ϳ�����Ȼ����ˮ�����������̼�����´���Ӧ�õ�CO��H2���õ���CO��H2�Ļ��������Ϊ�ϳ�������ͼΪ���úϳ����ϳ�����ȼ�϶����ѣ�CH3OCH3����ұ�����IJ����������̣� ��1�����ø�¯β���е�ˮ���������������õ��ϳ�������ѧ����ʽΪ
��1�����ø�¯β���е�ˮ���������������õ��ϳ�������ѧ����ʽΪ