��Ŀ����
 ��2011?������һģ��һ̼��ѧ���Է�����ֻ��һ��̼ԭ�ӵĻ������CO��CH4�ȣ�Ϊԭ�����ϳ�һϵ�л���ԭ�Ϻ�ȼ�ϵĻ�ѧ����ҵ�ϳ�����Ȼ����ˮ�����������̼�����´���Ӧ�õ�CO��H2���õ���CO��H2�Ļ��������Ϊ�ϳ�������ͼΪ���úϳ����ϳ�����ȼ�϶����ѣ�CH3OCH3����ұ�����IJ����������̣�
��2011?������һģ��һ̼��ѧ���Է�����ֻ��һ��̼ԭ�ӵĻ������CO��CH4�ȣ�Ϊԭ�����ϳ�һϵ�л���ԭ�Ϻ�ȼ�ϵĻ�ѧ����ҵ�ϳ�����Ȼ����ˮ�����������̼�����´���Ӧ�õ�CO��H2���õ���CO��H2�Ļ��������Ϊ�ϳ�������ͼΪ���úϳ����ϳ�����ȼ�϶����ѣ�CH3OCH3����ұ�����IJ����������̣���1����������ƾ���C2H5OH���Ļ�ѧʽ���
��ͬ
��ͬ
��ѡ���ͬ������ͬ�������������ڿ�������ȫȼ�����ɶ�����̼��ˮ�û�ѧ��Ӧ����ʽΪCH3OCH3+3O2
2CO2+3H2O
| ||
CH3OCH3+3O2
2CO2+3H2O
�����ڶ����ѻ����Ϸ�һ�״ɰ壬�ɹ۲쵽�״ɰ�����
| ||
ˮ��
ˮ��
����2����ԭ��Ӧ���ڷ����Ļ�ѧ��Ӧ�������������ʲ��μӷ�Ӧ������
2
2
��������ԭ��Ӧ�����ü�����ȫȼ��ά�ָ��£���ԭ��Ӧ�����ò�Ʒ������
������
��ѡ����ڡ������ڡ�����������3��д������Ӧ���з����Ļ�ѧ��Ӧ����ʽ
CH4+H2O
CO+3H2
| ||
CH4+H2O
CO+3H2
����дһ�������������������У��ɲ���ѭ����������
| ||
CO2
CO2
��H2O
H2O
����4����ɫ��ѧ���ص�֮һ�ǡ����ŷš�������Ӧ��ԭ��ȫ�����뵽��Ҫ�IJ�Ʒ�У��ɺϳ����Ʊ������ѵķ�Ӧ
����
����
��ѡ����ϡ��������ϡ�����һ�ص㣮��5�������������������úϳ�����CO��H2�������Ȳ�������
AC
AC
��A��14��3 B��7��1 C��14��5 D��28��5��
��������1���Ƚ϶�������ƾ��Ļ�ѧʽ��ԭ�ӵ����༰���������ݶ�����ȼ�յķ�Ӧд����Ӧ�ķ���ʽ�����ݶ�����ȼ�յIJ������ʵ�������
��2�����ݻ�ԭ��Ӧ�������ʵ����ʷ��������ķ�Ӧ������ԭ��Ӧ�����ü�����ȫȼ��ά�ָ��£���ԭ��Ӧ�����ò�Ʒ����̼��
��3�����ݴ���Ӧ���з����ķ�Ӧд����Ӧ�ķ���ʽ���������úϳ����ϳ�����ȼ�϶����ѣ�CH3OCH3����ұ�����IJ����������̷�������ѭ�������ʣ�
��4�����������ϳ����ڵķ�Ӧ����CO��H2�������ȵķ�Χ��
��2�����ݻ�ԭ��Ӧ�������ʵ����ʷ��������ķ�Ӧ������ԭ��Ӧ�����ü�����ȫȼ��ά�ָ��£���ԭ��Ӧ�����ò�Ʒ����̼��
��3�����ݴ���Ӧ���з����ķ�Ӧд����Ӧ�ķ���ʽ���������úϳ����ϳ�����ȼ�϶����ѣ�CH3OCH3����ұ�����IJ����������̷�������ѭ�������ʣ�
��4�����������ϳ����ڵķ�Ӧ����CO��H2�������ȵķ�Χ��
����⣺��1���ɶ�������ƾ��Ļ�ѧʽ��ԭ�ӵ����༰������ͬ��֪����������ƾ���C2H5OH���Ļ�ѧʽ�����ͬ���������ڿ�������ȫȼ�����ɶ�����̼��ˮ����Ӧ�ķ���ʽΪ��CH3OCH3+3O2
2CO2+3H2O�����ڶ�����ȼ�յIJ�������ˮ�����ԣ����ڶ����ѻ����Ϸ�һ�״ɰ壬�ɹ۲쵽�״ɰ�����ˮ�飻
��2����ԭ��Ӧ���ڷ����Ļ�ѧ��Ӧ��������һ����̼����������������Ӧ������ԭ��Ӧ�����ü�����ȫȼ��ά�ָ��£������ɵ����в�����̼��������������
��3���ڴ���Ӧ����ˮ���������������õ�������һ����̼����ѧ��Ӧ����ʽΪ��CH4+H2O
CO+3H2�������������п�֪���ɲ���ѭ����������CO2��H2O��
��4���ϳ����Ʊ������ѵķ�Ӧ���е�ԭ��ȫ��ת��Ϊ�������У��������ŷŵ��ص㣻
��5�������������з����ķ�Ӧ��֪����CH4+H2O
CO+3H2������CO��H2��������Ϊ28��6=14��3����CO2+CH4
2CO+2H2������CO��H2��������Ϊ56��4=14��1�����ԣ������������������úϳ�����CO��H2����������14��1��14��3֮�䣮���ԣ���������AC��
�ʴ�Ϊ����1����ͬ��CH3OCH3+3O2
2CO2+3H2O��ˮ�飻��2��2�������ڣ���3��CH4+H2O
CO+3H2��CO2��H2O����4�����ϣ���5��AC��
| ||
��2����ԭ��Ӧ���ڷ����Ļ�ѧ��Ӧ��������һ����̼����������������Ӧ������ԭ��Ӧ�����ü�����ȫȼ��ά�ָ��£������ɵ����в�����̼��������������
��3���ڴ���Ӧ����ˮ���������������õ�������һ����̼����ѧ��Ӧ����ʽΪ��CH4+H2O
| ||
��4���ϳ����Ʊ������ѵķ�Ӧ���е�ԭ��ȫ��ת��Ϊ�������У��������ŷŵ��ص㣻
��5�������������з����ķ�Ӧ��֪����CH4+H2O
| ||
| ||
�ʴ�Ϊ����1����ͬ��CH3OCH3+3O2
| ||
| ||
�������������Ĺؼ��Ǹ�������ṩ����Ϣ���ҳ���Ӧ������д����Ӧ�ķ���ʽ���ٸ��ݷ���ʽ�����й�����ķ������жϣ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
 ��2011?������һģ���ں�ۡ��ۺͷ���֮�佨����ϵ�ǻ�ѧѧ�Ƶ��ص㣮
��2011?������һģ���ں�ۡ��ۺͷ���֮�佨����ϵ�ǻ�ѧѧ�Ƶ��ص㣮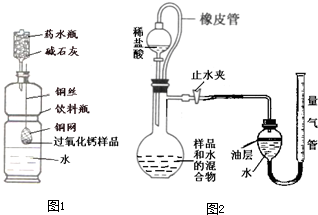 ��2011?������һģ����;��������ʱ�����dz���ˮ�м��������������ƣ�CaO2�����壬Ϊ���ṩ����������������ˮ��Ӧ�����������⣬������ʲô���ʣ�������ȤС���������һ�����н���̽����
��2011?������һģ����;��������ʱ�����dz���ˮ�м��������������ƣ�CaO2�����壬Ϊ���ṩ����������������ˮ��Ӧ�����������⣬������ʲô���ʣ�������ȤС���������һ�����н���̽����