��Ŀ����
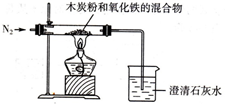 ��֪ľ̿�ۺ�Fe2O3��Ӧ�Ļ�ѧ����ʽ��2Fe2O3+3C
��֪ľ̿�ۺ�Fe2O3��Ӧ�Ļ�ѧ����ʽ��2Fe2O3+3C
| ||
��1��ʵ��ǰ������Ҫ���еIJ�����
��ͨ��������װ���в���Ŀ���
��ͨ��������װ���в���Ŀ���
����2��ʵ���У�ʢ�ų���ʯ��ˮ���ձ��г��ֵ�������
����ʯ��ˮ�����
����ʯ��ˮ�����
��ԭ������Ӧ�����˴����Ķ�����̼
��Ӧ�����˴����Ķ�����̼
����3��ʵ����ͨ��������Ǵ��������N2��д�����ÿ��������ɣ�
��
������������������ȼ������ᷢ����ը����Σ��
������������������ȼ������ᷢ����ը����Σ��
����
�����к��кܶຬ��Ԫ�ص����ʣ���������Ŷ�����������Ԫ�غ������ж�
�����к��кܶຬ��Ԫ�ص����ʣ���������Ŷ�����������Ԫ�غ������ж�
��Ҳ�����ó�ʪ�ĵ�����ԭ����
Ӱ������������Ԫ�غ����IJⶨ
Ӱ������������Ԫ�غ����IJⶨ
����4������3.2g��������̼��ַ�Ӧ����ó���ʯ��ˮ����1.32g��������������Ԫ�ص���������Ϊ
30%
30%
����������1�������Ҫ��Ҫ��ʵ��Ŀ�Ŀ��ǣ�
��2������ȷ����⣬��֪������̼��ʯ��ˮ��Ӧ��������Լ�����������ʣ�
��3���������ʵ���Ҫ���ע������濼�ǣ�
��4��ʯ��ˮ���ӵ�������Ϊ���ɵ�CO2���������ɷ���ʽ3C+2Fe2O3
4Fe+3CO2����֪������������Ԫ�ص�������Ϊ���ɵ�CO2����Ԫ�ص�����������Ԫ�ص�����������ʽ���㼴�ɣ�
��2������ȷ����⣬��֪������̼��ʯ��ˮ��Ӧ��������Լ�����������ʣ�
��3���������ʵ���Ҫ���ע������濼�ǣ�
��4��ʯ��ˮ���ӵ�������Ϊ���ɵ�CO2���������ɷ���ʽ3C+2Fe2O3
| ||
����⣺��1�������е������ܹ���̼������Ӧ���ɶ�����̼�����ʣ�Ϊ�˷�ֹ����ʵ����ۣ���Ӧ��ͨ�뵪�����Ͼ����ڿ�����Ȼ���ڼ��ȣ�
��2����Ϊľ̿�ۺ���������Ӧ�����ɵĶ�����̼����ͨ�����ʯ��ˮ���ձ��У���ʯ��ˮ������Ӧ������̼��Ƴ��������Կ��Թ۲쵽�����ʯ��ˮ����ǣ�
��3����Ϊ�����к��ж�����̼������������ʹ��Ӧ�����������������Ӷ�����ʵ������ȷ��
ʵ����Ҳ�����ó�ʪ�ĵ�����ԭ���ǣ��ó�ʪ�ĵ�����ʹ�ȵ��Թ�ը�ѣ�ͬʱ�ڸ�����ˮҲ�ܺ�̼��Ӧ������ľ̿��������������Ӧ���ᵼ�²ⶨ����Ԫ�ص���������ƫ��
��4��ʯ��ˮ���ӵ�������Ϊ���ɵ�CO2���������ɷ���ʽ3C+2Fe2O3
4Fe+3CO2����֪������������Ԫ�ص�������Ϊ���ɵ�CO2����Ԫ�ص�������CO2����Ԫ�ص�����Ϊ1.32g��
��100%=0.96g��������������Ԫ�ص���������Ϊ��
��100%=30%��
�ʴ�Ϊ����1����ͨ��������װ���в���Ŀ�����
��2������ʯ��ˮ����ǣ���Ӧ�����˴����Ķ�����̼��
��3���ٻ�����������������ȼ������ᷢ����ը����Σ�գ�
�ڿ����к��кܶຬ��Ԫ�ص����ʣ���������Ŷ�����������Ԫ�غ������жϣ�Ӱ������������Ԫ�غ����IJⶨ��
��4��30%��
��2����Ϊľ̿�ۺ���������Ӧ�����ɵĶ�����̼����ͨ�����ʯ��ˮ���ձ��У���ʯ��ˮ������Ӧ������̼��Ƴ��������Կ��Թ۲쵽�����ʯ��ˮ����ǣ�
��3����Ϊ�����к��ж�����̼������������ʹ��Ӧ�����������������Ӷ�����ʵ������ȷ��
ʵ����Ҳ�����ó�ʪ�ĵ�����ԭ���ǣ��ó�ʪ�ĵ�����ʹ�ȵ��Թ�ը�ѣ�ͬʱ�ڸ�����ˮҲ�ܺ�̼��Ӧ������ľ̿��������������Ӧ���ᵼ�²ⶨ����Ԫ�ص���������ƫ��
��4��ʯ��ˮ���ӵ�������Ϊ���ɵ�CO2���������ɷ���ʽ3C+2Fe2O3
| ||
| 16��2 |
| 12+16��2 |
| 0.96g |
| 3.2g |
�ʴ�Ϊ����1����ͨ��������װ���в���Ŀ�����
��2������ʯ��ˮ����ǣ���Ӧ�����˴����Ķ�����̼��
��3���ٻ�����������������ȼ������ᷢ����ը����Σ�գ�
�ڿ����к��кܶຬ��Ԫ�ص����ʣ���������Ŷ�����������Ԫ�غ������жϣ�Ӱ������������Ԫ�غ����IJⶨ��
��4��30%��
������������Ҫ����ѧ����ʵ�������Ҫ���ע���������������Լ�����Ԫ�ص�����������ʽ���м����������

��ϰ��ϵ�д�
�����Ŀ
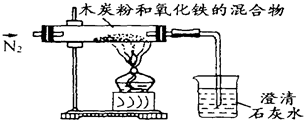 ��֪ľ̿�ۺ�Fe2O3 ��Ӧ�Ļ�ѧ����ʽΪ��2Fe2O3+3C
��֪ľ̿�ۺ�Fe2O3 ��Ӧ�Ļ�ѧ����ʽΪ��2Fe2O3+3C ��֪ľ̿�ۺ�Fe2O3��Ӧ�Ļ�ѧ����ʽ��2Fe2O3+3C
��֪ľ̿�ۺ�Fe2O3��Ӧ�Ļ�ѧ����ʽ��2Fe2O3+3C 4Fe+CO2����ijͬѧ�����һ��ʵ�飬֤���������к�����Ԫ�أ����ⶨ����������Ԫ�ص�����������ʵ��װ����ͼ��
4Fe+CO2����ijͬѧ�����һ��ʵ�飬֤���������к�����Ԫ�أ����ⶨ����������Ԫ�ص�����������ʵ��װ����ͼ�� 4Fe+CO2����ijͬѧ�����һ��ʵ�飬֤���������к�����Ԫ�أ����ⶨ����������Ԫ�ص�����������ʵ��װ����ͼ��
4Fe+CO2����ijͬѧ�����һ��ʵ�飬֤���������к�����Ԫ�أ����ⶨ����������Ԫ�ص�����������ʵ��װ����ͼ��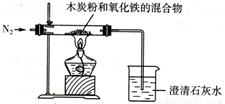
 4Fe+3CO2��������Fe2O3+3C
4Fe+3CO2��������Fe2O3+3C 2Fe+3CO��
2Fe+3CO�� 4FeOʮCO2����Ϊ�Ƶ����ֻ�����ߵĴ�����Ӧ��480g Fe2O3��ĩ�м���̼���ٿˣ� ��
4FeOʮCO2����Ϊ�Ƶ����ֻ�����ߵĴ�����Ӧ��480g Fe2O3��ĩ�м���̼���ٿˣ� ��