��Ŀ����
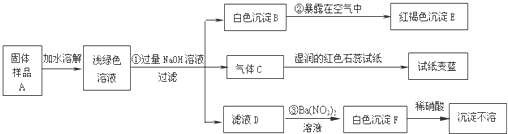
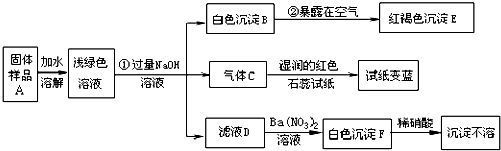
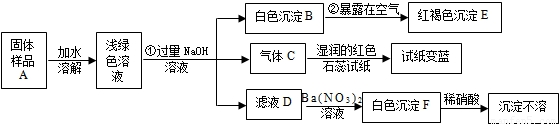
29��ij���½�һ��������һ�ֲ�Ʒ���ڡ����Ρ����ò�Ʒ��������������鵵Ȼ�ľ�ķ��ϣ�ij��ѧ������ȤС��̽���ò�Ʒ����ɣ����������µ�ʵ�飺���������ϻ�֪�������Ρ���ָ�����������Ӻ�һ�����������ɵ��Σ����±ʯ��KCl?MgCl2?6H2O������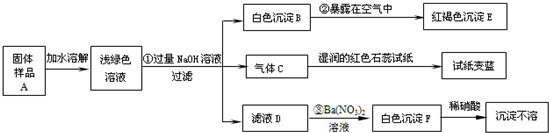
�Իش��������⣺
��1��д���������ʵĻ�ѧʽ��C
��2��д�����б仯�Ļ�ѧ����ʽ��
��
��3������̽�����˵����ƷA�к��е�������

�Իش��������⣺
��1��д���������ʵĻ�ѧʽ��C
NH3
��EFe��OH��3
��FBaSO4
��2��д�����б仯�Ļ�ѧ����ʽ��
��
4Fe��OH��2+O2+2H2O�T4Fe��OH��3
��Na2SO4+Ba��NO3��2�TBaSO4��+2NaNO3
��3������̽�����˵����ƷA�к��е�������
Fe2+��NH4+��SO42-
������������dz��ɫ��Һ���������ӵ���Һ�����ɫ����������������������ɫ������������������ܽ⣬˵���ð�ɫ�������������ᱵ���Ȼ��������������ٽ��������Ϣ���о���������ɵó����ۣ�
����⣺��1������C��ʹʪ��ĺ�ɫʯ����ֽ������˵���������Լ��ԣ�����Ϊ�Ǽ����������Ƶõ��ļ������壬��������C�ǰ��������ɫ������������������������ɫ������������������ܽ⣬˵���ð�ɫ�������������ᱵ���Ȼ�������������Ϊ�Ǽ������ᱵ��õ��İ�ɫ������û�г��������ӣ�����ɫ���������ᱵ��
��2������dz��ɫ��Һ�к����������ӣ����Լ����������ƺ�õ�������������ɫ���������Եڢڸ���Ӧ�ķ�Ӧ����������������������ˮ������������������������ż����ƽ���ɣ��ɼ������ᱵ��õ��İ�ɫ���������������ɫ�������ܽ⣬˵���ð�ɫ���������ᱵ������˵����ҺD�к�����������ӣ�����Ϊǰ������˹������������ƣ������������������ӣ�������ҺD�к��������ƣ����Եڢ۸���Ӧ�ķ�Ӧ���������ƺ����ᱵ�������������ᱵ�������ƣ��ù۲취��ƽ���ɣ�
��3��������Ʒ����ˮ�У��õ�dz��ɫ��Һ˵��������Ʒ�к����������ӣ�����C��ʹʪ��ĺ�ɫʯ����ֽ������˵���������Լ��ԣ�����Ϊ�Ǽ����������Ƶõ��ļ������壬��������C�ǰ�����������������笠����ӽ���ܲ�������������˵��������Ʒ�к���笠����ӣ��ɼ������ᱵ��õ��İ�ɫ���������������ɫ�������ܽ⣬˵���ð�ɫ���������ᱵ������˵����ҺD�к�����������ӣ�˵��������Ʒ�к�����������ӣ�
�ʴ�Ϊ����1��NH3��Fe��OH��3��BaSO4��
��2����4Fe��OH��2+O2+2H2O�T4Fe��OH��3
��Na2SO4+Ba��NO3��2�TBaSO4��+2NaNO3
��3��Fe2+��NH4+��SO42-��
��2������dz��ɫ��Һ�к����������ӣ����Լ����������ƺ�õ�������������ɫ���������Եڢڸ���Ӧ�ķ�Ӧ����������������������ˮ������������������������ż����ƽ���ɣ��ɼ������ᱵ��õ��İ�ɫ���������������ɫ�������ܽ⣬˵���ð�ɫ���������ᱵ������˵����ҺD�к�����������ӣ�����Ϊǰ������˹������������ƣ������������������ӣ�������ҺD�к��������ƣ����Եڢ۸���Ӧ�ķ�Ӧ���������ƺ����ᱵ�������������ᱵ�������ƣ��ù۲취��ƽ���ɣ�
��3��������Ʒ����ˮ�У��õ�dz��ɫ��Һ˵��������Ʒ�к����������ӣ�����C��ʹʪ��ĺ�ɫʯ����ֽ������˵���������Լ��ԣ�����Ϊ�Ǽ����������Ƶõ��ļ������壬��������C�ǰ�����������������笠����ӽ���ܲ�������������˵��������Ʒ�к���笠����ӣ��ɼ������ᱵ��õ��İ�ɫ���������������ɫ�������ܽ⣬˵���ð�ɫ���������ᱵ������˵����ҺD�к�����������ӣ�˵��������Ʒ�к�����������ӣ�
�ʴ�Ϊ����1��NH3��Fe��OH��3��BaSO4��
��2����4Fe��OH��2+O2+2H2O�T4Fe��OH��3
��Na2SO4+Ba��NO3��2�TBaSO4��+2NaNO3
��3��Fe2+��NH4+��SO42-��
�����������ƶ���ʱҪ�ҳ�ͻ�ƿڣ�����ѧ��ȥ��֪ʶ���������DZ��⣬֪��dz��ɫ��Һ��������������Һ�����ɫ�����������������������������а�����������ɫ��Һ��ͭ������Һ������������İ�ɫ���������ᱵ���Ȼ������ֳ�����

��ϰ��ϵ�д�
 ��ĩ���ƾ�ϵ�д�
��ĩ���ƾ�ϵ�д� ���ɿ��ñ���ϵ�д�
���ɿ��ñ���ϵ�д�
�����Ŀ