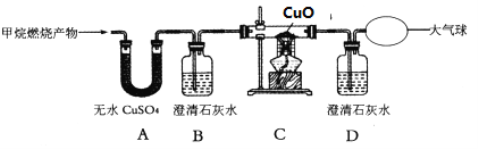
��Ŀ����
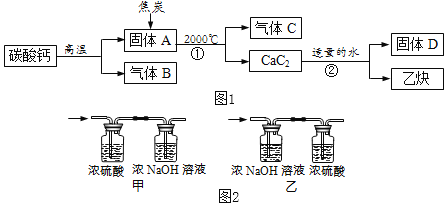
����Ŀ����ҵ��������ʯ(CaC2)���Ʊ���Ҫ��ҵԭ����Ȳ(CxHy)������ͼ1��ʾ��
�����ϡ�
��̼��Ƹ��·ֽ�ɵ����������
��Ũ�������ǿ��ˮ�ԣ�NaOH��Һ������CO2��
�����ۡ�
(1)C��B���Ԫ����ͬ��C�ж�����Ӧ�ٻ�ѧ����ʽΪ_____��
(2)D����ˮ���ܽ�����¶����߶���С��D�Ļ�ѧʽ��_____��
���ⶨ��Ȳ��ɡ�
���ϣ�һ����Ȳ�������ĸ�ԭ�ӹ��ɡ�
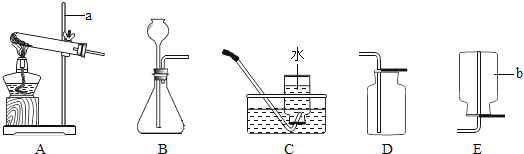
(3)��һ��������Ȳ(CxHy)��ȫȼ�գ��������ɵ����建��ͨ����ͼ2��ʾ��_____(ѡ����������������)װ�ã����ٻ���ͨһ��ʱ���N2������¼ʵ������������С�
װ�� | ��Ӧǰ���� | ��Ӧ������ |
Ũ���� | 125.3g | 127.1g |
ŨNaOH��Һ | 78.2g | 87.0g |
(4)ʵ����������ͨһ��ʱ���N2����Ŀ����_____��
(5)���㣺��Ȳ��̼Ԫ������Ϊ_____g����Ԫ������Ϊ_____g����Ȳ��ѧʽΪ_____��
(6)��Ӧ�ڵĻ�ѧ����ʽΪ_____��
���𰸡� CaO+3C![]() CaC2+CO�� Ca(OH)2 �� ʹ���ɵ�ˮ�Ͷ�����̼ȫ������Ӧװ����ȫ���� 2.4 0.2 C2H2 CaC2+2H2O�TCa(OH)2+C2H2��
CaC2+CO�� Ca(OH)2 �� ʹ���ɵ�ˮ�Ͷ�����̼ȫ������Ӧװ����ȫ���� 2.4 0.2 C2H2 CaC2+2H2O�TCa(OH)2+C2H2��
����������1��CaCO3![]() CaO+CO2������B��CO2��C��B���Ԫ����ͬ��C�ж�����C��CO����ѧ��Ӧ���������غ㶨�ɣ��������⣬��Ӧ�ٻ�ѧ����ʽΪ��CaO��3C
CaO+CO2������B��CO2��C��B���Ԫ����ͬ��C�ж�����C��CO����ѧ��Ӧ���������غ㶨�ɣ��������⣬��Ӧ�ٻ�ѧ����ʽΪ��CaO��3C![]() CaC2��CO����2��D����ˮ���ܽ�����¶����߶���С����D��Ca(OH)2����3����Ȳ��ȫȼ�����ɶ�����̼��ˮ��NaOH��Һ������CO2��Ũ�������ǿ��ˮ�ԣ�����������ͨ��Ũ���ᣬŨ�������ӵ���������Ȳȼ������ˮ����������ͨ��NaOH��Һ�����ӵ���������Ȳȼ�����ɶ�����̼�������������ͨ��NaOH��Һ����Ӱ����Ȳȼ������ˮ����������ѡ�ס���4��ʵ���С�����ͨһ��ʱ���N2����Ŀ����ʹ���ɵ�ˮ�Ͷ�����̼ȫ������Ӧװ����ȫ���ա���5����ѧ��Ӧǰ�����Ԫ�ص�������ȣ���Ȳ��̼Ԫ�ص������������ɵĶ�����̼��̼Ԫ�ص���������87.0g-78.2g����
CaC2��CO����2��D����ˮ���ܽ�����¶����߶���С����D��Ca(OH)2����3����Ȳ��ȫȼ�����ɶ�����̼��ˮ��NaOH��Һ������CO2��Ũ�������ǿ��ˮ�ԣ�����������ͨ��Ũ���ᣬŨ�������ӵ���������Ȳȼ������ˮ����������ͨ��NaOH��Һ�����ӵ���������Ȳȼ�����ɶ�����̼�������������ͨ��NaOH��Һ����Ӱ����Ȳȼ������ˮ����������ѡ�ס���4��ʵ���С�����ͨһ��ʱ���N2����Ŀ����ʹ���ɵ�ˮ�Ͷ�����̼ȫ������Ӧװ����ȫ���ա���5����ѧ��Ӧǰ�����Ԫ�ص�������ȣ���Ȳ��̼Ԫ�ص������������ɵĶ�����̼��̼Ԫ�ص���������87.0g-78.2g����![]() =2.4g,��Ȳ����Ԫ�������������ɵ�ˮ����Ԫ�ص���������127.1g-125.3g����
=2.4g,��Ȳ����Ԫ�������������ɵ�ˮ����Ԫ�ص���������127.1g-125.3g����![]() =0.2g������Ȳ�Ļ�ѧʽΪCxHy ,��
=0.2g������Ȳ�Ļ�ѧʽΪCxHy ,��![]() =
=![]() �����
�����![]() =1���л���������ǰ�̼ԭ�Ӹ������ٵ����˳������Ϊ��ij����ij����Ȼ����Ȳ����̼ԭ��Ϊ2��������Ȳ�Ļ�ѧʽΪC2H2��6����ѧ��Ӧǰ��ԭ������䡢����ԭ�Ӹ�����ȣ��ʷ�Ӧ�ڵĻ�ѧ����ʽ��CaC2��2H2O=Ca(OH)2��C2H2��
=1���л���������ǰ�̼ԭ�Ӹ������ٵ����˳������Ϊ��ij����ij����Ȼ����Ȳ����̼ԭ��Ϊ2��������Ȳ�Ļ�ѧʽΪC2H2��6����ѧ��Ӧǰ��ԭ������䡢����ԭ�Ӹ�����ȣ��ʷ�Ӧ�ڵĻ�ѧ����ʽ��CaC2��2H2O=Ca(OH)2��C2H2��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�