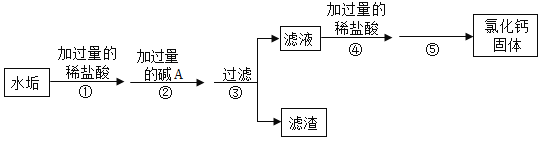
��Ŀ����
����Ŀ��ʵ����ϣ�ͬѧ�Ƿ���һƿ���õ��������ƹ����ƿ���а�ɫ��ĩ������ʦָ���£�ͬѧ�ǶԸð�ɫ��ĩ�ijɷֽ�����̽����
����������裩�����ɫ��ĩ��̼����
�����ɫ��ĩ����������
�����ɫ��ĩ��__________________________________��
���������ϣ�CaCl2��Һ��Ca(NO3)2��Һ�����ԡ�
��ʵ��̽����ȡ��ɫ��ĩ����ˮ�γ���ҺA���������ʵ�飺
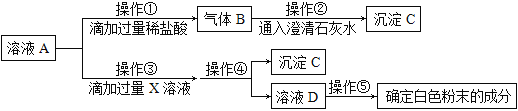
��1��������ʵ���֪������_______�϶�������������C�Ļ�ѧʽ__________________��
��2�������ٲ�������Ļ�ѧ��Ӧ����ʽ�ǣ�_______________________________________��
��3�������۵�X��Һ���������µ�_______________������ĸ��ţ���
A CaCl2��Һ B Ca(OH)2��Һ C Ba(OH)2��Һ D Ca(NO3)2��Һ
��4�������ܵ�����___________________________��
��5����д�������ݵ�ʵ�����£�
���� | ���� | ���� |
����һ����ȡ������ҺD�����ɫ��ΰ�Ŀ�Ѩ�У��ٵμ���ɫ��̪��Һ | _______________________ | �������� |
���������ڽྻ�IJ���Ƭ�Ϸ�һƬpH��ֽ���� _____________________________����pH��ֽ�ϣ�����ֽ��ʾ����ɫ�����ɫ���Ƚϣ�����pH�� | pH��7 | ����____���� |
���𰸡�̼���ƺ��������� �� CaCO3 2HCl+Na2CO3=2NaCl+H2O+CO2�� BC ���� ��Һ����ɫ �ò�����պȡ��ҺD ��
��������
[���������]����1����ȫ���ʣ���ɫ��ĩ��̼���ƣ������û�б��ʣ���ɫ��ĩ���������ƣ����Բ�����ֱ��ʣ���ɫ��ĩ��̼���ƺ��������ƣ�
��1��������ʵ���֪������ϡ����ʱ�������壬˵����Һ�к���̼���ƣ������϶���������������̼���������Ʒ�Ӧ����̼��Ƴ�����ˮ��C�Ļ�ѧʽΪCaCO3��
��2�������̼���Ʒ�Ӧ���ɶ�����̼���Ȼ��ƺ�ˮ����Ӧ�Ļ�ѧ����ʽΪ��2HCl+Na2CO3=2NaCl+H2O+CO2����
��3�����˲����۵�X��Һ���Ȼ�����Һ���������Һ��Ca(OH)2��Һ�� Ba(OH)2��Һ�Լ��ԣ�������������Ƶļ���,��ѡBC��
��4���������ǽ�������Һ����룬�����ǹ��ˣ�
��5������һ����ȡ������ҺD�����ɫ��ΰ�Ŀ�Ѩ�У��ٵμ���ɫ��̪��Һ����Һ����ɫ��˵��ֻ��̼���ƣ�����������
���������ڽྻ�IJ���Ƭ�Ϸ�һƬpH��ֽ���ò�����պȡ��ҺD����pH��ֽ�ϣ�����ֽ��ʾ����ɫ�����ɫ���Ƚϣ��õ�pH��7������������

����Ŀ����ѧ��ȤС��ͬѧ����ʵ��̨�ϰ�����˳��ڷ���7ƿ��ͬ����ɫ��Һ����ͼ��ʾ��������4��5���Լ�ƿ��ǩ����
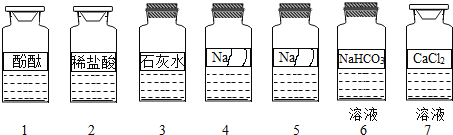
��������⣩����ƿ�Լ��ijɷֱַ���ʲô��
���������ϣ��ټ��Ե��������ƣ�Na2SO3����Һ��Na2CO3��Һ�����Ե�CaCl2��Һ���ɷ������ֽⷴӦ��������ɫ������
��Na2SO3+2HCl=2NaCl+SO2��+H2O��
��CO2��SO2������ʹ����ʯ��ˮ����ǣ�
���������룩���������ǩ����Ϣ���Ʋ�4��5����Һ���ֱܷ���NaOH��Һ��Na2CO3��Һ��Na2SO3��Һ��NaCl��Һ�е�һ�֣�
��ʵʩ��������ȡ����4��5����Һ���Թ��У��ֱ������������ʵ�飮
ʵ����� | ʵ������ | ʵ����� |
ʵ��1
| ��Һ������ɫ��ɺ�ɫ | 4��5����Һ�������������������е�__��Һ�� |
ʵ��2
| ��������ɫ���� | 4��5����Һ���ֱܷ���Na2SO3��Һ��Na2CO3��Һ�е�һ�֣�д������һ����Ӧ�Ļ�ѧ����ʽ __�� |
������ʵ�飩Ϊ�˽�һ��ȷ��4��5����Һ�ijɷ֣�ͬѧ�Ƿֱ�ȡ����4��5����Һ���Թ��м���ʵ�飮
ʵ����� | ʵ������ | ʵ����� |
| __ | 4����Һ��Na2CO3��Һ 5����Һ��Na2SO3��Һ�� |
��ʵ�鷴˼��ͬѧ�Ǿ���������Ϊ4����Һ�������DZ��ʵ�NaOH��Һ���������������ʵ�鷽��������֤�����������ʵ�鱨�森
ʵ����� | ʵ������ | ʵ����� |
__ | __ | 4����Һ�Dz��ֱ��ʵ�NaOH��Һ�� |