��Ŀ����
����Ŀ���ҹ�����������ȫ�������ḻ����ҵ�ϴӴӺ��н���Ԫ�صĿ�ʯ���������������г���������������Ҫԭ�ϡ���ش��������⣺
I������Ʒ�㷺Ӧ���������������С�
��1�����в��������Ͻ����_____________(ѡ����ĸ)��
A��Fe(OH)3 B������ C�������
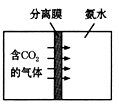
��2���������⣬����_____�仯(ѡ�����������ѧ��)����ͼ��̽�������ڲ�ͬ�����·��������ʵ�飬��������ʴ������______(ѡ����ĸ)��
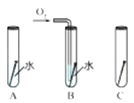
II��������(��Ҫ�ɷ�ΪFe2O3)����������������
��3����Fe2O3�У���Ԫ�ص���������Ϊ_____________��
��4���������ᴿ��õ��Ĵ���Fe2O3����������ij�ֹ�ҵ����(��Ҫ�ɷ�ΪFeO��Fe2O3)���䷴Ӧԭ��Ϊ��2 Fe2O3+C![]() 4FeO+CO2�����ֽ���̿������Fe2O3��Ͼ��ȣ�������ԭ����ַ�Ӧ����ͼΪ��������������淴Ӧʱ��ı仯���ߡ�
4FeO+CO2�����ֽ���̿������Fe2O3��Ͼ��ȣ�������ԭ����ַ�Ӧ����ͼΪ��������������淴Ӧʱ��ı仯���ߡ�
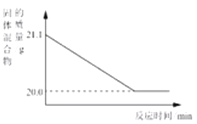
����ͼ��֪������CO2���������Ϊ_____g��
�����㷴Ӧ�����������FeO����������(����ݻ�ѧ����ʽд�������ļ��㲽��)��
���𰸡���1��A����2����ѧ��B����3��30%����4����1.1����36%��
����������1���Ͻ����ڽ��������ۺ������Ľ�����ǽ����Ƶõľ��н������������ʣ��ʺϽ�����������������������������Ͻ𣬹�ѡA����2���������Ҫ�ɷ�����������������������������������ѧ�仯�������������������ˮ������ͬʱ�Ӵ�����ͼ��֪������Bͼ���������⣻��3��Fe2O3�У���Ԫ�ص���������=![]() 30%��
30%��
��4�����������غ㶨�ɻ�ѧ��Ӧǰ�����ʵ����������䣬�ʼ��ٵ�������Ϊ���ɵĶ�����̼�����������ɵĶ�����̼������=21.1g-20.0g=1.1g��
���û�ѧ����ʽ���������������������̼��Ӧ�������ȼ��ɼ��������������������
�����ɵ���������������Ϊx��
2 Fe2O3+C![]() 4FeO+CO2��
4FeO+CO2��
288 44
X 1.1g
![]() x=7.2g
x=7.2g
��Ӧ�����������FeO����������=![]() 36%
36%
�𣺷�Ӧ�����������FeO����������Ϊ36% ��

����Ŀ����l���������й�CO2�����ܽ�ȵļ������ݣ�
CO2��ˮ�е��ܽ�ȣ���λ��ml��
ѹǿ/����ѹ | �¶�/�� | ||||
0 | 25 | 50 | 75 | 100 | |
1 | 1.79 | 0.752 | 0.423 | 0.307 | 0.231 |
10 | 15.92 | 7.14 | 4.095 | 2.99 | 2.28 |
25 | 29.30 | 16.20 | 9.71 | 6.82 | 5.73 |
��������ݷ�����
�� ����CO2��ˮ�е��ܽ�ȱ�������������������Ӱ�������ܽ�ȵģ�_____��______��
�� ����ƿ�����������˵��ԭƿ�е�ѹǿ_____���С�ڡ����ڻ���ڣ�ƿ�����ѹǿ��
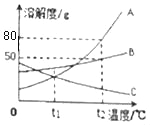
��2������ͼ���ᾧˮ����Ĺ��������ˮ�е��ܽ�����ߣ�����ݸ�����ͼ�ش��������⣺
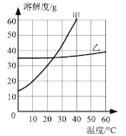
�� 20��ʱ��150gˮ���ܽ�______g������ǡ���γɱ�����Һ��
�� ����60�����ҵı�����Һ�������Һ�йص����У�
A��ˮ������ B����Һ�����ʵ����� C����Һ������
D���ҵ��������� E��60��ʱ�ҵ��ܽ��
�����¶Ȳ��䣬���ñ�����Һϡ�ͣ���������ǣߣ�����ţ���ͬ����������ñ�����Һ������20�������������_________��