��Ŀ����
����Ŀ����l���������й�CO2�����ܽ�ȵļ������ݣ�
CO2��ˮ�е��ܽ�ȣ���λ��ml��
ѹǿ/����ѹ | �¶�/�� | ||||
0 | 25 | 50 | 75 | 100 | |
1 | 1.79 | 0.752 | 0.423 | 0.307 | 0.231 |
10 | 15.92 | 7.14 | 4.095 | 2.99 | 2.28 |
25 | 29.30 | 16.20 | 9.71 | 6.82 | 5.73 |
��������ݷ�����
�� ����CO2��ˮ�е��ܽ�ȱ�������������������Ӱ�������ܽ�ȵģ�_____��______��
�� ����ƿ�����������˵��ԭƿ�е�ѹǿ_____���С�ڡ����ڻ���ڣ�ƿ�����ѹǿ��
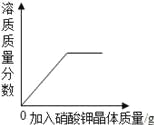
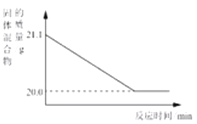
��2������ͼ���ᾧˮ����Ĺ��������ˮ�е��ܽ�����ߣ�����ݸ�����ͼ�ش��������⣺
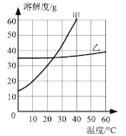
�� 20��ʱ��150gˮ���ܽ�______g������ǡ���γɱ�����Һ��
�� ����60�����ҵı�����Һ�������Һ�йص����У�
A��ˮ������ B����Һ�����ʵ����� C����Һ������
D���ҵ��������� E��60��ʱ�ҵ��ܽ��
�����¶Ȳ��䣬���ñ�����Һϡ�ͣ���������ǣߣ�����ţ���ͬ����������ñ�����Һ������20�������������_________��
���𰸡� ��1�����¶����ߣ�������ܽ�ȼ�С��ѹǿ����������ܽ�������ش�������ɸ��֣������ڣ���2����45����BE��AE
����������1�����ɱ����е����ݿ�֪��ѹǹ��ͬ��ʱ���¶����ߣ�������ܽ�ȼ�С���¶���ͬʱ��ѹǿ����������ܽ�������ش�������ɸ��֣��ڢ� ����ƿ�����������˵��ԭƿ�е�ѹǿ����ƿ�����ѹǿ������2�����ܽ����һ���¶��£�100g�ܼ���ﵽ����ʱ�����ܽ�����ʵ���������ͼ��֪��20��ʱ�����ܽ��Ϊ30g��150gˮ���ܽ�45g������ǡ���γɱ�����Һ�����ܽ����һ���¶��£�100g�ܼ���ﵽ����ʱ�����ܽ�����ʵ�������������Һ���ʵ���������=�ܽ�������ܽ��+100g����100% ��60�����ҵı�����Һ��˵��ÿ140g������Һ����40g���ң�����������Ϊ40g����40g+100g����100%=28.6%�������¶Ȳ��䣬���ñ�����Һϡ�ͣ�������������ʵ�������ͬʱ�����¶Ȳ��䣬�ܽ��Ҳ���䣻������ñ�����Һ������20�����ܽ�ȱ�С�����������������ܼ��������䣬���ʵ�����������С����Һ���������١�

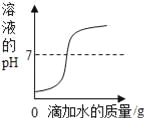
����Ŀ������ͼ�����й����ı仯������ȷ���ǣ� ��
|
|
|
|
A��ij�¶��£���һ�����������������Һ�в��ϼ�������ؾ��� | B����һ������ϡ��������εμ�ˮ | C����һ�������������ƺ�̼���ƵĻ����Һ����εμ����� | D����ˮͨ����һ��ʱ�� |
A. A B. B C. C D. D
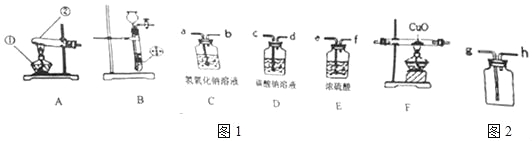
����Ŀ���ҹ�������ѧ�Һ�°����������ˡ������Ƽ������ԭ������Ҫ��һ������ʳ��ˮ���Ⱥ�ͨ������NH3��CO2�Ʊ�NaHCO3����ѧ����ʽ��NaCl+ NH3+CO2+H2O= NaHCO3��+NH4Cl
ij��ȤС����ʵ����ģ��ù��̣����Ͼ���IJ���ش��������⣺
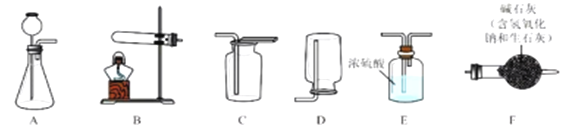
I�������Ʊ�
��1��������̼�����Ʊ�
ʵ���ҳ�����ʯ��ʯ��ϡ���ᷴӦ�Ʊ�CO2���仯ѧ����ʽΪ_____________��Ӧѡ��������ռ�װ��Ϊ______(ѡ��װ�ö�Ӧ����ĸ)��
��2�������Ʊ�
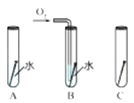
ʵ���ҳ����������հ�������ֹ��Ⱦ������ʵ�����Ʊ�NH3�ķ�Ӧԭ��Ϊ��Ca(OH)2(��)+2NH4Cl(��) ![]() CaCl2+2H2O+2NH3������Ҫ�Ʊ�������NH3����ѡװ�õ���ȷ����˳��Ϊ_____��______(ѡ��װ�ö�Ӧ����ĸ)��ʵ�����Ʊ�O2Ҳ���������Ʊ�NH3�ķ���װ�ã�д���ø÷���װ���Ʊ�O2�Ļ�ѧ����ʽ__________��
CaCl2+2H2O+2NH3������Ҫ�Ʊ�������NH3����ѡװ�õ���ȷ����˳��Ϊ_____��______(ѡ��װ�ö�Ӧ����ĸ)��ʵ�����Ʊ�O2Ҳ���������Ʊ�NH3�ķ���װ�ã�д���ø÷���װ���Ʊ�O2�Ļ�ѧ����ʽ__________��
II��NaHCO3�Ʊ�
���� | NaHCO3 | NH4Cl |
�ܽ��/g(20��) | 9.6 | 37.2 |
��3�����ݱ����е��ܽ�����ݣ�����20��������NaHCO3�ܹ��ȴ���Һ�нᾧ������ԭ��________��
��4���ù�������һ����NH4Cl��ũҵ�����г�������______________��