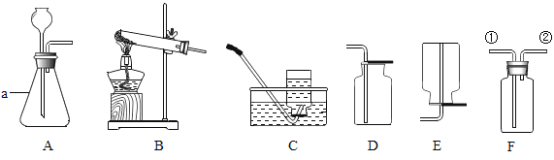
��Ŀ����
����Ŀ��ij�С�������ͼ��ʾ����ģ��������ʵ�飬���Բ���ijɷֽ���̽����
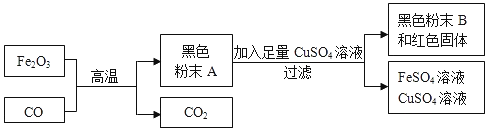
��������⣩��ɫ��ĩA������ʲô�أ�
�����в��룩��1��С����Ϊ��ɫ��ĩAȫ�������ۡ���д��������CuSO4��Һ��Ӧ�Ļ�ѧ����ʽ_________________________________��ʵ������в�ȡ�˹��ˣ��ò������õ�������������������_____________��
��2��С����ΪС���IJ����Ǵ���ģ�������__________________��
��3��С������˲���ٺͲ���ڣ�������ѧ֪ʶ�������µ�һ�����롣
����٣���ɫ��ĩA�����ۺ�������������Fe3O4�����
����ڣ���ɫ��ĩA�����ۺ�����������FeO����ɣ�
����ۣ�_____________________________��
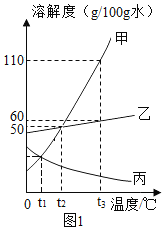
���������ϣ����������ﶼ����ϡ���ᣬϡ���ᷴӦ������������Ϊ����ɫ�����Ϊ��ɫ������ֻ�������������ܱ�����������
��ʵ��̽����
���� | ʵ����� | ���ܵ����� | ���� |
�� | ȡ������ɫ��ĩA �ô������� | ��ɫ��ĩȫ�������� | �������ȷ |
�� | ȡ������ɫ��ĩA �ô������� | ��ɫ��ĩ���ֱ����� | �������ȷ |
�� | ȡ������ɫ��ĩB��������ϡ���� | ��ɫ��ĩȫ���ܽ� | �������ȷ |
�� | ________________ | __________________ | �������ȷ |
����˼���ۣ�С����Ϊ�����ںͷ������еó��Ľ��۶�����ȷ����������__________________ ��
���ó����ۣ��ڸ����£�һ����̼����������Ӧ����������������������������������д��ֻ�������������ķ���ʽ_________________________��
���𰸡�Fe��CuSO4=FeSO4��Cu ���� ����ɫ��ĩAȫ�������ۣ����������������ͭ��Һ����ͭ������������Һ��һ��û�к�ɫ��ĩBʣ�� ��ɫ��ĩA�����ۡ������������������������ ȡ������ɫ��ĩB���ô������� ��ɫ��ĩ���ֱ����� �������У�ȡ������ɫ��ĩA���ô�����������ɫ��ĩ���ֱ����������ɫ��ĩA�п��ܺ����������������������У�ȡ������ɫ��ĩB��������ϡ���ᣬ���������������������������ܱ������ܽ⣬����˵����ɫ��ĩ�������ۺ����������� 2CO��Fe2O3![]() Fe��FeO��2CO2
Fe��FeO��2CO2
��������
���в��룺
��1��С����Ϊ��ɫ��ĩAȫ�������ۡ�������CuSO4��Һ��Ӧ��������������ͭ�Ļ�ѧ����ʽ��Fe��CuSO4=FeSO4��Cu��ʵ������в�ȡ�˹��ˣ��ò������õ������������������ǣ���������ֹҺ��ɽ���
��2��С����ΪС���IJ����Ǵ���ģ������ǣ�����ɫ��ĩAȫ�������ۣ����������������ͭ��Һ����ͭ������������Һ��һ��û�к�ɫ��ĩBʣ�ࣻ
��3����ɫ��ĩA�����ۺ�������������ɣ����ɫ��ĩA�����ۺ�����������ɣ����ɫ��ĩA�����ۡ�����������������������ɣ��ʲ���ۣ���ɫ��ĩA�����ۡ�����������������������ɣ�
ʵ�鷽���ܣ�ȡ������ɫ��ĩB���ô�����������ɫ��ĩ���ֱ�������˵����ɫ��ĩA�����ۡ�����������������������ɣ��������ȷ��
��˼���ۣ�
С����Ϊ�����ںͷ������еó��Ľ��۶�����ȷ���������ǣ��������У�ȡ������ɫ��ĩA���ô�����������ɫ��ĩ���ֱ����������ɫ��ĩA�п��ܺ���������������
�������У�ȡ������ɫ��ĩB��������ϡ���ᣬ���������������������������ܱ������ܽ⣬����˵����ɫ��ĩ�������ۺ�����������
�ó����ۣ�
�ڸ����£�һ����̼����������Ӧ�������������������Ͷ�����̼�Ļ�ѧ����ʽ��2CO��Fe2O3![]() Fe��FeO��2CO2��
Fe��FeO��2CO2��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ��ij��ѧ��ȤС���ͬѧ��ѧϰ���꼶����ѧ���²��е����Ͽ�Ƭ��ʯ�������ʯ���γ���ʱ������������ˮ��̼��Ƶ��������ж�����̼��ˮʱ���ᷴӦ�����ܽ��Խϴ��̼����ƣ�CaCO3+CO2+H2O�TCa(HCO3)2�����뵽ʵ�����г���ʯ��ˮ�������̼��Ӧ������̼��ƣ�Ca(OH)2+CO2�TCaCO3��+H2O���Գ�ʱ�������Һ��ͨ��CO2����Ӧ����Һ�е�������ɲ�����Ũ�����Ȥ��
��������⣩һ����CO2��NaOH��Һ��Ӧ������������ʲô��
���������ϣ�
��1��ͨ������CO2��Ӧ�Ļ�ѧ����ʽΪ______��
��2��ͨ�����CO2����Ӧ�Ļ�ѧ����ʽΪNa2CO3+CO2+H2O�T2NaHCO3��
��3��̼�����ζ��ǿ�����ˮ�ģ�BaCO3������ˮ��
��4��̼��������Һ�ʼ��ԡ�
��������룩
��1������ΪNaOH��Na2CO3��
��2������ΪNa2CO3��
��3������Ϊ______���ѧʽ����
��4������ΪNaHCO3��
�����ʵ�飩
ʵ�鲽�� | ʵ������ | ʵ����� |
��1���ò�����պȡ��Ӧ����Һ������pH��ֽ�� | pH=9 | ����Һ�� ______ �� |
��2��ȡ��Ӧ����Һ�������Թ��У������еμӹ�����BaCl2��Һ | �� ______ ���� | ���루4�������� |
��3��ȡ���裨2���е��ϲ���Һ������ϡ���� | ������ð�� | ���� ______ ������ |
���ó����ۣ����루3��������
�����۽�����
��1����ͬѧ�����ʵ�鲽�裨1���Ƕ���ġ�����Ϊ��ʵ������Ƿ���Ҫ��______��������Ҫ����������Ҫ������
��2��ͬѧ����һ�ΰ�Ŀ��Ͷ���˽̲ģ��������Ȼ�ѹǿ��Сʱ��Ca(HCO3)2�TCaCO3��+CO2��+H2O����������ɷ����������NaHCO3���ķ�Ӧԭ����֮���ƣ���д��NaHCO3���ȷֽ�Ļ�ѧ����ʽ��______ ��
����˼Ӧ�ã������ʯ��ˮ�в���ͨ�������̼���۲쵽��������______��