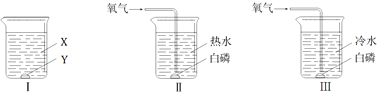
ΧβΡΩΡΎ»ί
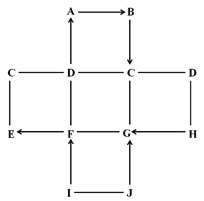
ΓΨΧβΡΩΓΩ»γΆΦΥυ ΨΒΡ «Έο÷ AΓΪFœύΜΞΦδΒΡΙΊœΒ,ΆΦ÷–ΓΑΓζΓ±±μ ΨΈο÷ Φδ¥φ‘ΎΒΡΉΣΜ·ΙΊœΒΘ§ΓΑ--Γ±±μ ΨΝΫΕΥΒΡΈο÷ ΡήΖΔ…ζΜ·―ßΖ¥”ΠΓΘAΓΔBΓΔCΓΔDΓΔE «≥θ÷–Μ·―ß≥ΘΦϊΒΡΈε÷÷≤ΜΆ§άύ±πΒΡΈο÷ Θ§Τδ÷–AΈΣΚλ…ΪΙΧΧεΘ§B «…ζ≤ζΓΔ…ζΜν÷–Ήν≥ΘΦϊΒΡΒΡΫπ τ≤ΡΝœΘΜ DΈΣ»ΥΧε÷–ΈΗΥαΒΡ÷ς“Σ≥…Ζ÷ΘΜ FΙψΖΚ”Ο”Ύ≤ΘΝßΓΔ‘λ÷ΫΓΔΖΡ÷·ΚΆœ¥Β”ΦΝΒΡ…ζ≤ζΒ»ΓΘ
«κΜΊ¥πΘΚ
Θ®1Θ©FΒΡΜ·―ß Ϋ_______Θ§CΩ…Ρή «_______ΓΘ
Θ®2Θ©–¥≥ωEΒΡ“Μ÷÷”ΟΆΨ_________________________________________ΓΘ
Θ®3Θ©–¥≥ωœ¬Ν–Μ·―ßΖ¥”ΠΖΫ≥Χ ΫΘΚ
AΓζB____________________________________________ΓΘ
FΓζE____________________________Θ§ΗΟΖ¥”ΠΒΡΜυ±Ψάύ–Ά «___________ΓΘ
ΓΨ¥πΑΗΓΩ Na2CO3 CuSO4ΜρCuCl2ΜρCu(NO3)2 ¬·ΨΏ«εΫύΦΝ/»Ξ≥ΐ”ΆΈέ/”Ο”ΎΖ ‘μΓΔ ·”ΆΓΔ‘λ÷ΫΓΔΖΡ÷·ΚΆ”Γ»ΨΒ»ΙΛ“Β Fe2O3+3CO![]() 2Fe+3CO2 Na2CO3+CaΘ®OHΘ©2=2Na0H+CaCO3Γΐ Η¥Ζ÷ΫβΖ¥”Π
2Fe+3CO2 Na2CO3+CaΘ®OHΘ©2=2Na0H+CaCO3Γΐ Η¥Ζ÷ΫβΖ¥”Π
ΓΨΫβΈωΓΩB «…ζ≤ζΓΔ…ζΜν÷–Ήν≥ΘΦϊΒΡΒΡΫπ τ≤ΡΝœΘ§‘ρBΈΣΧζΘΜΡ«Ο¥Κλ…ΪΙΧΧεAΈΣ―θΜ·ΧζΘΜDΈΣ»ΥΧε÷–ΈΗΥαΒΡ÷ς“Σ≥…Ζ÷Θ§‘ρD «―ΈΥαΘΜFΙψΖΚ”Ο”Ύ≤ΘΝßΓΔ‘λ÷ΫΓΔΖΡ÷·ΚΆœ¥Β”ΦΝΒΡ…ζ≤ζΒ»Θ§‘ρF «ΧΦΥαΡΤΓΘF”κEΡήœύΜΞΉΣΜ·Θ§EΓΔD÷°ΦδΡήΖΔ…ζΜ·―ßΖ¥”ΠΘ§‘ρEΩ…Ρή ««β―θΜ·ΡΤΘΜCΦ»Ρή”κEΖ¥”ΠΘ§”÷Ρή”κBΖ¥”ΠΘ§‘ρCΩ…Ρή «Ά≠―ΈΓΘ
ΫβΘΚΘ®1Θ©FΒΡΜ·―ß ΫNa2CO3Θ§CΩ…Ρή «CuSO4ΜρCuCl2ΜρCu(NO3)2ΘΜ
Θ®2Θ©E ««β―θΜ·ΡΤΘ§EΒΡ“Μ÷÷”ΟΆΨ «ΘΚ¬·ΨΏ«εΫύΦΝ/»Ξ≥ΐ”ΆΈέ/”Ο”ΎΖ ‘μΓΔ ·”ΆΓΔ‘λ÷ΫΓΔΖΡ÷·ΚΆ”Γ»ΨΒ»ΙΛ“ΒΘΜ
Θ®3Θ©–¥≥ωœ¬Ν–Μ·―ßΖ¥”ΠΖΫ≥Χ ΫΘΚAΓζBΘΚFe2O3+3CO ΗΏΈ¬2Fe+3CO2ΘΜ
FΓζEΘΚNa2CO3+ Ca(OH)2=2Na0H+CaCO3ΓΐΘΜΗΟΖ¥”ΠΒΡΜυ±Ψάύ–Ά «ΘΚΗ¥Ζ÷ΫβΖ¥”ΠΓΘ

ΓΨΧβΡΩΓΩΦχ±πœ¬Ν–ΗςΉιΈο÷ ΒΡΖΫΖ®≤Μ’ΐ»ΖΒΡ «
―Γœν | Φχ±πΒΡΈο÷ | Φχ±πΒΡΖΫΖ® |
A | ¥ΩΥ°ΚΆΩσ»ΣΥ° | Ιέ≤λ «Ζώ≥Έ«ε |
B | Εΰ―θΜ·ΧΦΚΆ“Μ―θΜ·ΧΦ | Βψ»ΦΘ§Ιέ≤λ «ΖώΡή»Φ…’ |
C | Υ°ΚΆΙΐ―θΜ·«β»ή“Κ | Φ”Εΰ―θΜ·ΟΧΘ§Ιέ≤λ «Ζώ”–Τχ≈ί |
D | ±υΚΆΗ…±υ | “Έ¬Θ®20ΓφΘ©Ζ≈÷ΟΘ§Ιΐ“ΜΕΈ ±ΦδΙέ≤λ «Ζώ”–“ΚΧε≤–Ντ |
A. A B. B C. C D. D