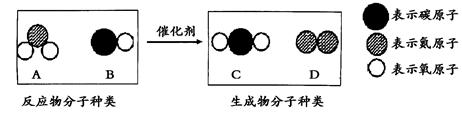
��Ŀ����
������ѧ�Ļ�ѧ֪ʶ���
(1)��ɳ���Խ�˵���ƽ�����Ȼ������Ҫ�� (����ʡ�������)��ʽ���ڣ�
(2)������ٺ���ġ�������Һ��������ʯ�ҽ�������ͭ��Һ��϶��ɣ��û�Ϲ����з�Ӧ�Ļ�ѧ����ʽΪ��_____________________________���ѡ�������Һ�����䵽�����ϳ��ֵ���ɫ�ߵ�����ó�������________ϴ����
(3)��װ���Ĵ�����𣬿�����ˮ��ȴ���塢����ɳ��Χ��������������ȼ�յĻ�ѧ����ʽΪ�� ��������ԭ���� �� ��
(1)��ɳ���Խ�˵���ƽ�����Ȼ������Ҫ�� (����ʡ�������)��ʽ���ڣ�
(2)������ٺ���ġ�������Һ��������ʯ�ҽ�������ͭ��Һ��϶��ɣ��û�Ϲ����з�Ӧ�Ļ�ѧ����ʽΪ��_____________________________���ѡ�������Һ�����䵽�����ϳ��ֵ���ɫ�ߵ�����ó�������________ϴ����
(3)��װ���Ĵ�����𣬿�����ˮ��ȴ���塢����ɳ��Χ��������������ȼ�յĻ�ѧ����ʽΪ�� ��������ԭ���� �� ��
(1) ����
(2) CuSO4+Ca(OH)2===Cu(OH)2��+CaSO4�� ʳ��
(3) 4P+5O2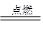 2P2O5 ���¶Ƚ��������Ż�����£���������������Ӵ�
2P2O5 ���¶Ƚ��������Ż�����£���������������Ӵ�
(2) CuSO4+Ca(OH)2===Cu(OH)2��+CaSO4�� ʳ��
(3) 4P+5O2
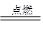 2P2O5 ���¶Ƚ��������Ż�����£���������������Ӵ�
2P2O5 ���¶Ƚ��������Ż�����£���������������Ӵ���1��ɳ���Խ�˵�������Ե��ʵ���ʽ������ɳ�У���Ϊ��Ļ�ѧ���ʺܲ����ã�
��2�����ݸ��ֽⷴӦ�ķ���������֪��ʯ�ҽ�������ͭ��Һ��������������ͭ����ɫ����������ƣ�������ͭ�Ǽ���Ժͳ����е���������ʳ�����кͷ�Ӧ����������
��3������ȼ�������ȼ�ղ�����ͬ�����������������ף������Ż�ˮ��ȴ�����ǽ����¶ȡ�����ɳ��Χ�������Ǹ���������
��2�����ݸ��ֽⷴӦ�ķ���������֪��ʯ�ҽ�������ͭ��Һ��������������ͭ����ɫ����������ƣ�������ͭ�Ǽ���Ժͳ����е���������ʳ�����кͷ�Ӧ����������
��3������ȼ�������ȼ�ղ�����ͬ�����������������ף������Ż�ˮ��ȴ�����ǽ����¶ȡ�����ɳ��Χ�������Ǹ���������

��ϰ��ϵ�д�
�����Ŀ