��Ŀ����
��10�֣����������߽���ѧ���ӻ�ѧ������ᡱ�������С��һ��ش��������⡣
��1��Т�����ˣ����л�����Ĵ�ͳ���¡����ؾ��꼶ѧ��С�졢С������ʮ��Ϊ����Ժ��ȥ�����ˮ�ɻ���������Ժ�����÷��ӵ����ʽ����ŵ������ԭ��_________________��
��2��С���ڱ����������̿����Ч�س�ȥ��ζ��������������̿��___________�ԡ�
��3��С��ҵ����г�����Ʒ�У����ڽ������ϵ���______�����ţ���




A.������ B.��ɰ�� C.�������̨ D.���ʿ���
��4��С�췢�ּ��з������ϵ���Ƥ���⼣�߰ߣ���ͬ�ڰ�װ�������������������������������������������ԭ��_________________________________________________��
��5��������ζƷ������һ��ʧȥ��ǩ�İ�ɫ���壬������ʳ�Σ�Ҳ�����Ǵ��Ϊ���ж���ɷ֣�С���������ʵ������������ʵ�飺

���жϴ˰�ɫ������_____________________��д��ѧʽ����д����Ӧ�ڵĻ�ѧ����ʽΪ
________________________________________________��
��6��С��ҳ�������泥���ѧʽΪNH4NO3����������ķ��ϡ����������___�����ţ���
A.���� B.��
C.�ط� D.���Ϸ�
��1��Т�����ˣ����л�����Ĵ�ͳ���¡����ؾ��꼶ѧ��С�졢С������ʮ��Ϊ����Ժ��ȥ�����ˮ�ɻ���������Ժ�����÷��ӵ����ʽ����ŵ������ԭ��_________________��
��2��С���ڱ����������̿����Ч�س�ȥ��ζ��������������̿��___________�ԡ�
��3��С��ҵ����г�����Ʒ�У����ڽ������ϵ���______�����ţ���




A.������ B.��ɰ�� C.�������̨ D.���ʿ���
��4��С�췢�ּ��з������ϵ���Ƥ���⼣�߰ߣ���ͬ�ڰ�װ�������������������������������������������ԭ��_________________________________________________��
��5��������ζƷ������һ��ʧȥ��ǩ�İ�ɫ���壬������ʳ�Σ�Ҳ�����Ǵ��Ϊ���ж���ɷ֣�С���������ʵ������������ʵ�飺

���жϴ˰�ɫ������_____________________��д��ѧʽ����д����Ӧ�ڵĻ�ѧ����ʽΪ
________________________________________________��
��6��С��ҳ�������泥���ѧʽΪNH4NO3����������ķ��ϡ����������___�����ţ���
A.���� B.��
C.�ط� D.���Ϸ�
��1�������ڲ����˶� ��2������ ��3��C
��4������Ʒ��������Ӧ�������γ�һ�����ܵ�����������Ĥ�Ⱥ�����
��5��Na2CO3��д���Ʋ����֣� Ca(OH)2+CO2=CaCO3��+H2O ��6��A
��4������Ʒ��������Ӧ�������γ�һ�����ܵ�����������Ĥ�Ⱥ�����
��5��Na2CO3��д���Ʋ����֣� Ca(OH)2+CO2=CaCO3��+H2O ��6��A
��1���ŵ���ζ����Ϊ���ӵIJ����˶�����2������̿��ˮ�����������������ԣ�������ˮ�е�һЩ��ζ�����ʣ���3����������ڽ������ϣ���4�����Ļ�ѧ���ʱȽϻ��ã��ܺ�������Ӧ����һ�����ܵ������ﱡĤ����ֹ���������ĽӴ��������������ã���5����ɫ�����������������壬�ʰ�ɫ����Ϊ̼���ƣ���6������泥�NH4NO3���к���ֲ�������Ӫ��Ԫ��--��Ԫ�أ������ڵ��ʣ�

��ϰ��ϵ�д�
�����Ŀ
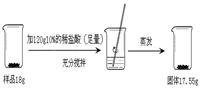

 Pb��2H2SO4��PbO2��PbO2��Pb�Ļ��ϼ�Ϊ_______��
Pb��2H2SO4��PbO2��PbO2��Pb�Ļ��ϼ�Ϊ_______��