��Ŀ����
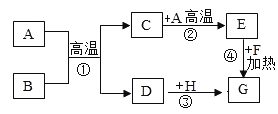
����Ŀ�� (7��)���¿�ͼ�е����ʾ�Ϊ���л�ѧ�еij������ʣ����мס��ҡ������������Ϊ���ʣ������£���Ϊ��ɫ���壬��Ϊ�Ϻ�ɫ������ũҵ�ϳ���F�������������������£�G��һ�־��д̼�����ζ�����壬��ˮ��Һ�Լ��ԣ���ҵ���üͱ������Ʊ�G��
��1��д������B��H�Ļ�ѧʽ��
�� B H
��2��C��E��ѧ���ʲ�ͬ������Ϊ��
��3��д��A�ֽ�Ϊ���ҵĻ�ѧ����ʽ��___________________
��4��д��F��G�Ļ�ѧ����ʽ��________
��5���ͱ������Ʊ�G���ͱ�������֮��Ϊ��
���𰸡���1��C CaCO 3 CuO ��2�����ӹ��ɲ�ͬ ��3��2H2O![]() 2H2��+O2��
2H2��+O2��
��4��Ca(OH) 2+(NH4) 2SO4![]() CaSO4+2H2O +2NH3�� ��5��3:14
CaSO4+2H2O +2NH3�� ��5��3:14
��������
���������������Ϊ�ƶ��⣬��Ҫ�ļ��������ҳ���Ŀ��ͻ�ƿڣ�����������ȷ�������ʣ�Ȼ�����ȷ������������ȷ�����ɣ�������Ҫ�����ҵ�����Ϊ�Ϻ�ɫ����Ϊͭ����Ϊ��ɫ����Ϊ̼��ũҵ�ϳ���F������������Ϊ�������ƣ�G��һ�־��д̼�����ζ�����壬��ˮ��Һ�Լ���һ��Ϊ������Ȼ�������ص����ʺͱ仯��������ȷ����

��ϰ��ϵ�д�
�����Ŀ