��Ŀ����
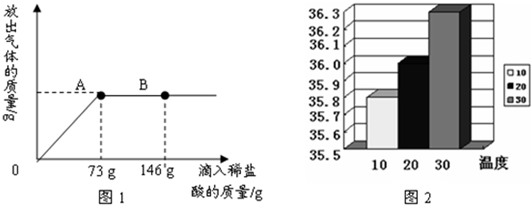
��һ������ Na2CO3�����������ˮ�ܽ⣬���59.2g ��Һ���������μ�������������Ϊ20%��ϡ���ᣬ�ų������������������ϡ�����������ϵ��ͼ1��ʾ����Ӧ�����Һ�¶�Ϊ20�棩���������ش��������⣮
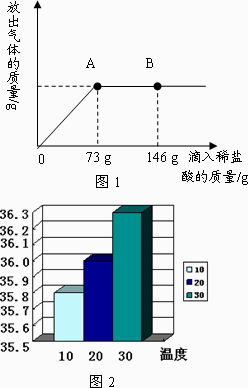
����������ϣ���ͬ�¶����Ȼ��Ƶ��ܽ�ȣ���ͼ2��
��1����Ӧ��ȫʱ���ų������������Ϊ______ g��
��2�����μ�ϡ������ͼ��B��ʱ���ձ�����Һ���������______��
��3�����μ���73gϡ����ʱ��ͨ������˵���ձ��е���Һ�Ƿ�Ϊ������Һ��
�⣺��1����ͼ����Կ������μ������������73gʱ��Ӧǡ����ɣ���ʱ������������Ϊ�������������
�������Ȼ��Ƶ�����Ϊx�����ɶ�����̼������Ϊy��
Na2CO3+2HCl�T2NaCl+H2O+CO2��
73 117 44
73g��10% x y
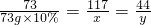
��ã�x=11.7g y=4.4g
��2����ͼʾ��֪�����μ�ϡ������ͼ��A��ʱ��������ȫ��Ӧ���ٵμ�ϡ������ͼ��B��ʱ���������ʣ�࣮����Һ�е�����ΪNaCl��HCl��
��3�����ݣ�1���еĽ�����֪����Ӧ����Һ�����ʼ��Ȼ��Ƶ�����Ϊ��11.7g��
��Һ��������Ϊ59.2g+73g-4.4g=127.8g��������Һ�е������ܹ�ȫ���ܽ⣬����Һ�����ʵ���������Ϊ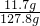 ��100%=9.1%��20��ʱ�Ȼ��Ƶ��ܽ��Ϊ36g���ʸı�����Һ��������������Ϊ
��100%=9.1%��20��ʱ�Ȼ��Ƶ��ܽ��Ϊ36g���ʸı�����Һ��������������Ϊ ��100%=26.5%�����Ը��¶��´�ʱ��Һ�����ͣ�
��100%=26.5%�����Ը��¶��´�ʱ��Һ�����ͣ�
�ʴ�Ϊ����1��4.4����2��NaCl��HCl����3���ձ��е���Һ��Ϊ���¶��µIJ�������Һ��
��������1�����������֪���μ�73gϡ����ʱ��ϡ�����̼����ǡ����ȫ��Ӧ��ʱ�̣����Ծݴ˽��ϡ���������������⣻
��2����ͼ�п��Կ���B��ʱϡ������������Դ�ʱ����Һ�е�����Ϊ�Ȼ��ƺ��Ȼ��⣬���Ծݴ˽��
��3�����ݣ�2���ķ�������֪��A��Ϊϡ�����̼����ǡ����ȫ��Ӧ��ʱ�̣����Կ��Ծݴ˼������Һ���Ȼ��Ƶ�����������Ȼ����ڸ��¶��µ��ܽ�Ƚ����жϼ��ɣ�
����������ϺõĿ���ѧ������ͼ����������ѧ��Ӧ��������ѧ��Ӧ��ȷͼ���еĹؼ��㼰�ߵı仯����ʾ�ĺ��壬��ͼ��ͻ�ѧ��Ӧ���ܽ���ǽ���Ĺؼ����ڣ�
�������Ȼ��Ƶ�����Ϊx�����ɶ�����̼������Ϊy��
Na2CO3+2HCl�T2NaCl+H2O+CO2��
73 117 44
73g��10% x y
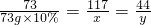
��ã�x=11.7g y=4.4g
��2����ͼʾ��֪�����μ�ϡ������ͼ��A��ʱ��������ȫ��Ӧ���ٵμ�ϡ������ͼ��B��ʱ���������ʣ�࣮����Һ�е�����ΪNaCl��HCl��
��3�����ݣ�1���еĽ�����֪����Ӧ����Һ�����ʼ��Ȼ��Ƶ�����Ϊ��11.7g��
��Һ��������Ϊ59.2g+73g-4.4g=127.8g��������Һ�е������ܹ�ȫ���ܽ⣬����Һ�����ʵ���������Ϊ
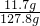 ��100%=9.1%��20��ʱ�Ȼ��Ƶ��ܽ��Ϊ36g���ʸı�����Һ��������������Ϊ
��100%=9.1%��20��ʱ�Ȼ��Ƶ��ܽ��Ϊ36g���ʸı�����Һ��������������Ϊ ��100%=26.5%�����Ը��¶��´�ʱ��Һ�����ͣ�
��100%=26.5%�����Ը��¶��´�ʱ��Һ�����ͣ��ʴ�Ϊ����1��4.4����2��NaCl��HCl����3���ձ��е���Һ��Ϊ���¶��µIJ�������Һ��
��������1�����������֪���μ�73gϡ����ʱ��ϡ�����̼����ǡ����ȫ��Ӧ��ʱ�̣����Ծݴ˽��ϡ���������������⣻
��2����ͼ�п��Կ���B��ʱϡ������������Դ�ʱ����Һ�е�����Ϊ�Ȼ��ƺ��Ȼ��⣬���Ծݴ˽��
��3�����ݣ�2���ķ�������֪��A��Ϊϡ�����̼����ǡ����ȫ��Ӧ��ʱ�̣����Կ��Ծݴ˼������Һ���Ȼ��Ƶ�����������Ȼ����ڸ��¶��µ��ܽ�Ƚ����жϼ��ɣ�
����������ϺõĿ���ѧ������ͼ����������ѧ��Ӧ��������ѧ��Ӧ��ȷͼ���еĹؼ��㼰�ߵı仯����ʾ�ĺ��壬��ͼ��ͻ�ѧ��Ӧ���ܽ���ǽ���Ĺؼ����ڣ�

��ϰ��ϵ�д�
 ��Ȥ����¹�֪��ϵ�д�
��Ȥ����¹�֪��ϵ�д� Ӣ��СӢ������Ĭдϵ�д�
Ӣ��СӢ������Ĭдϵ�д�
�����Ŀ
 23������֮�䷢����ѧ��Ӧʱ�������������Ե�������Щ��ѧ��Ӧȴ�۲첻�����Ե�����ij��ȤС��ͬѧΪ֤��NaOH��Һ��ϡ���ᷢ�����кͷ�Ӧ���Ӳ�ͬ�Ƕ����������ʵ�鷽����������ʵ�飮
23������֮�䷢����ѧ��Ӧʱ�������������Ե�������Щ��ѧ��Ӧȴ�۲첻�����Ե�����ij��ȤС��ͬѧΪ֤��NaOH��Һ��ϡ���ᷢ�����кͷ�Ӧ���Ӳ�ͬ�Ƕ����������ʵ�鷽����������ʵ�飮 ��2012?��ɽ��һģ������ͼ��װ�ý�һ������CO2��CO�Ļ��������з�����ͼ�е�a��b��c��d��Ϊ���������Կ��������ͨ����Һ��ļ��룬ʵ��ǰ�������ѹرգ���ѡ�����˵��Լ��������ʵ�飮��ѡ�õ��Լ��У���ϡ�����Ũ���������������Һ�ܳ����ʯ��ˮ���Լ���������
��2012?��ɽ��һģ������ͼ��װ�ý�һ������CO2��CO�Ļ��������з�����ͼ�е�a��b��c��d��Ϊ���������Կ��������ͨ����Һ��ļ��룬ʵ��ǰ�������ѹرգ���ѡ�����˵��Լ��������ʵ�飮��ѡ�õ��Լ��У���ϡ�����Ũ���������������Һ�ܳ����ʯ��ˮ���Լ���������