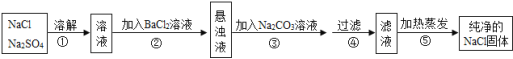��Ŀ����
����Ŀ���������������벻��ˮ��

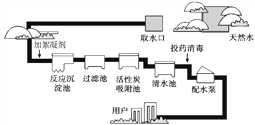
��1����Ȼ���ˮ�����Ǵ�ˮ������ˮ�����ó����� �������������ȷ�������ˮ��
��2��ȡijС������������ˮ��ֱ��ˮ���ֱ�����������ˮ�����裬����ֱ��ˮ���д�����ĭ��û�и���������ˮ����ĭ�����и�����˵��ֱ��ˮ��Ӳ�Ƚ� �����С������
��3���ճ�������ʹ��Ӳˮ����������鷳����ͥ�����м��ܽ���ˮ��Ӳ�ȣ�����ɱ�������ķ����� ��
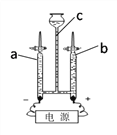
��4��ijͬѧ̽��ˮ����ɣ���ͼC��ʾ�����ˮ��ʵ�飮ʵ�鿪ʼ����ͬ����������ð����Ϊ����b�����壬��������ȼ�ŵ�ľ�������ܿڣ������۲쵽������ �����ˮ��Ӧ����ѧ����ʽΪ ��ʵ��֤��ˮ���� ��ɵģ�
���𰸡���1������ ��2��С ��3�����
��4�����屻��ȼ����������ɫ���� 2H2Oͨ��2H2�� + O2�� H��O
��������
�����������1��ˮ�ľ������̣����������ˡ�������������Ȼ���ˮ�����Ǵ�ˮ������ˮ�����ó����������������������ȷ�������ˮ
��2������������ˮ��ֱ��ˮ���ֱ�����������ˮ�����裬����ֱ��ˮ���д�����ĭ��û�и���������ˮ����ĭ�����и�����˵��ֱ��ˮ��Ӳ�Ƚ�С
��3���ճ�������ʹ��Ӳˮ����������鷳����ͥ�����м��ܽ���ˮ��Ӳ�ȣ�����ɱ�������ķ������
��4�����ˮʵ�飬���Դ�����������������������������Դ����������������������������������������������2������a�ܲ�������������b�ܲ�������������������b�����壬��������ȼ�ŵ�ľ�������ܿڣ������۲쵽���������屻��ȼ����������ɫ���������ˮ��Ӧ����ѧ����ʽΪ��2H2Oͨ��2H2�� + O2�������������غ㶨�ɣ���ѧ��Ӧǰ��Ԫ�ص�����䣬��ʵ��֤��ˮ����H��O��ɵ�

 ��У����ϵ�д�
��У����ϵ�д�