��Ŀ����
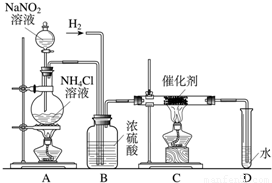
����������ʵ���ҳ��õ��Լ���ʵ����һ��������������Һ���Ȼ����Һ��Ӧ����ȡN2��N2�Ļ�ѧ����ʮ���ȶ�������һ������������H2���ֻ�������NH3����ͼΪ��ȡ����NH3��װ�ã���ȡH2��װ������ȥ����
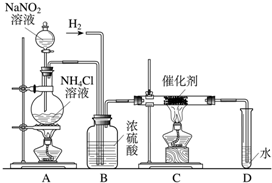
��1��Cװ�õ�Ӳ���Թ��з�����Ӧ�Ļ�ѧ����ʽΪ
��2����ӦʱN2��H2�������������
��3��B��Ũ�����������
��4����ʵ�鷽��֤��ȷʵ��NH3���ɣ�

��1��Cװ�õ�Ӳ���Թ��з�����Ӧ�Ļ�ѧ����ʽΪ
N2+3H2
2NH3
| ||
| �� |
N2+3H2
2NH3
��
| ||
| �� |
��2����ӦʱN2��H2�������������
14��3
14��3
��������˱������з�Ӧ����Ӧʱ��D�е��ܿ��Ƿ���ݳ����ݣ�����֪NH3��������ˮ����˵���ݳ����ݵ�ԭ����Ϊ�ǡ����ֻ��ϡ����Ի���δ��Ӧ��H2��N2�ݳ�
��Ϊ�ǡ����ֻ��ϡ����Ի���δ��Ӧ��H2��N2�ݳ�
����3��B��Ũ�����������
����
����
����4����ʵ�鷽��֤��ȷʵ��NH3���ɣ�
��D�е�����ɫ��̪��Һ����̪���
��D�е�����ɫ��̪��Һ����̪���
����������1�������������Ϣ��֪����Ӧ����NaNO2��NH4Cl����������N2��H20����������غ㶨�ɿ�֪�������ﻹ��NaCl��Ȼ������������C��������Ӧ���ɰ�����ע�ⷴӦ������
��2�������ʵ�����֮�ȵ�����Է��������͵ıȣ�������е������������ֻ��Ͽ�֪����������к��е�����������
��3������Ũ������ˮ�Է������ɣ�
��4�����鰱���ķ������Dz��ð�ˮ�ʼ��ԣ���Ϸ�̪���鼴�ɣ�
��2�������ʵ�����֮�ȵ�����Է��������͵ıȣ�������е������������ֻ��Ͽ�֪����������к��е�����������
��3������Ũ������ˮ�Է������ɣ�
��4�����鰱���ķ������Dz��ð�ˮ�ʼ��ԣ���Ϸ�̪���鼴�ɣ�
����⣺��1�����������غ㶨�ɼ������Ϣ��֪����Ӧ����NaNO2��NH4Cl����������N2��H20��NaCl��������NaNO2+NH4Cl
N2��+2H20+NaCl��������������C��������Ӧ���ɰ���N2+3H2
2NH3
��2�����û�ѧ����ʽ�����ʵ������ȵ������ԭ�������͵ı��ҵ������ʵ���������ȣ�
N2 +3H2
2NH3
14��2 6
���Ե���������������֮����14��3ʱ��Ϊ���ʣ�
֪����������к��е���������������������ˮ��û�������ų���
��4����Ϊ��ȡ����ʱ������ˮ���������ɣ������������Ϊ���
��5�����ǰ����ļ��鷽������Ϊ������ˮ��Һ�Լ��ԣ����Լ����̪��죮
�ʴ�Ϊ����1��3 H2+N2
2 NH3��2��14��3�� ��Ϊ�ǡ����ֻ��ϡ����Ի���δ��Ӧ��H2��N2�ݳ���3������
��4����D�е�����ɫ��̪��Һ����̪��죮
| ||
| ||
| �� |
��2�����û�ѧ����ʽ�����ʵ������ȵ������ԭ�������͵ı��ҵ������ʵ���������ȣ�
N2 +3H2
| ||
| �� |
14��2 6
���Ե���������������֮����14��3ʱ��Ϊ���ʣ�
֪����������к��е���������������������ˮ��û�������ų���
��4����Ϊ��ȡ����ʱ������ˮ���������ɣ������������Ϊ���
��5�����ǰ����ļ��鷽������Ϊ������ˮ��Һ�Լ��ԣ����Լ����̪��죮
�ʴ�Ϊ����1��3 H2+N2
| ||
| �� |
��4����D�е�����ɫ��̪��Һ����̪��죮
����������⿼��������ʵ�����Ʒ��������ļ��顢Ũ��������ʵ����ݣ�������ѧ֪ʶ��֪ʶ����Ǩ�Ƽ��ɽ�����⣮

��ϰ��ϵ�д�
 �¿α�������������ҵ�������γ�����ϵ�д�
�¿α�������������ҵ�������γ�����ϵ�д�
�����Ŀ
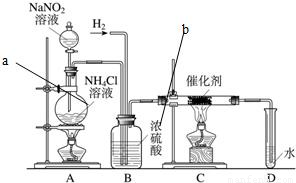
 ����������ʵ���ҳ��õ��Լ���ʵ����һ��������������Һ���Ȼ����Һ��Ӧ����ȡN2��N2�Ļ�ѧ����ʮ���ȶ�������һ������������H2���ֻ�������NH3����ͼΪ��ȡ����NH3��װ�ã���ȡH2��װ������ȥ����
����������ʵ���ҳ��õ��Լ���ʵ����һ��������������Һ���Ȼ����Һ��Ӧ����ȡN2��N2�Ļ�ѧ����ʮ���ȶ�������һ������������H2���ֻ�������NH3����ͼΪ��ȡ����NH3��װ�ã���ȡH2��װ������ȥ����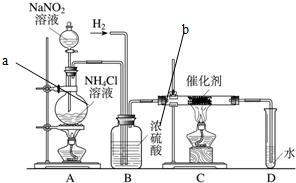 ����������ʵ���ҳ��õ��Լ���ʵ����һ��������������Һ���Ȼ����Һ��Ӧ����ȡN2��N2�Ļ�ѧ����ʮ���ȶ�������һ������������H2���ֻ�������NH3����ͼΪ��ȡ����NH3��װ�ã���ȡH2��װ������ȥ����
����������ʵ���ҳ��õ��Լ���ʵ����һ��������������Һ���Ȼ����Һ��Ӧ����ȡN2��N2�Ļ�ѧ����ʮ���ȶ�������һ������������H2���ֻ�������NH3����ͼΪ��ȡ����NH3��װ�ã���ȡH2��װ������ȥ����