��Ŀ����
ʵ������������õ���������Ϊ10%������������Һ���ܶ�Ϊ1.1g/cm3��������100g��������Ϊ5%������������Һ�����ô���Һ�ⶨij������Һ����������������
��1������100g��������Ϊ5%������������Һ����Ҫ10%������������Һ�� ��g��
��2����������������Һʱ����Ҫ�IJ����������� �����ιܡ��ձ�����������
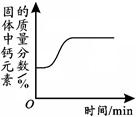
��3������õ�����������Һ�������������������������Һ��Ӧ��ʵ���������Һ��pH�仯������ͼ��ʾ��
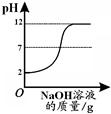
�ٸ�����ͼ�仯���ߣ��жϽ��еIJ������� ��������ĸ����
A����������Һ��εμӵ�����������Һ��
B��������������Һ��εμӵ�������Һ��
��b���Ӧ����Һ�е�����Ϊ�� ����д��ѧʽ����
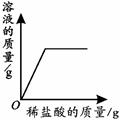
��4������ȫ�к�50gϡ������Һ��ǡ��������õ�����������Һ80g���Լ����ϡ���������������������д��������̣�
��1������100g��������Ϊ5%������������Һ����Ҫ10%������������Һ�� ��g��
��2����������������Һʱ����Ҫ�IJ����������� �����ιܡ��ձ�����������
��3������õ�����������Һ�������������������������Һ��Ӧ��ʵ���������Һ��pH�仯������ͼ��ʾ��
�ٸ�����ͼ�仯���ߣ��жϽ��еIJ������� ��������ĸ����
A����������Һ��εμӵ�����������Һ��
B��������������Һ��εμӵ�������Һ��
��b���Ӧ����Һ�е�����Ϊ�� ����д��ѧʽ����
��4������ȫ�к�50gϡ������Һ��ǡ��������õ�����������Һ80g���Լ����ϡ���������������������д��������̣�
��1��50����2����Ͳ����3����B����Na2SO4��NaOH����4��9.8%
�����������1������ϡ��ǰ�����ʲ��俼�ǡ�����Ҫ10%������������Һ����ΪX��100g��5%��X��10%���X��50g��
��2����ȡˮʱ��Ҫ�IJ�����������Ͳ����ͷ�ιܣ��ܽ�ʱ��Ҫ�IJ����������ձ��������������ȱ�ٲ�����������Ͳ��
��3����������ʾ����Ӧ����Һ������������˵����ʵ���ǰ�����������Һ��μ���������Һ�У���ʹ��Һ������������ΪB��
��b����Һ���ȴ���7��˵���μӵ��������ƹ����������ҺΪ���������������ƵĻ����Һ��������ΪNa2SO4 ��NaOH��
��4�����ݷ�Ӧ�Ļ�ѧ����ʽ����ǡ����ȫ��Ӧ�����������Ƶ�����������������50g������Һ���������������ø�������Һ�����ʵ��������������ϡ���������ʵ�����Ϊx
2NaOH+H2SO4 ��Na2SO4 +2H2O
80 98
80g��5% X
���ݣ�
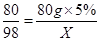
���X��4.9g
��ϡ�����������������Ϊ��
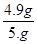 ��100%=9.8%
��100%=9.8%�𣺴����������������Ϊ9.8%

��ϰ��ϵ�д�
�����Ŀ