��Ŀ����
����Ŀ���ڹ�ҵ�ϣ�̼���ƹ㷺���ڲ�������ֽ����֯��ϴ�Ӽ��������ȡ�
�����Ͽ�Ƭ��
��1��̼�����׳ƴ��____���Դ������ʯ��Ϊԭ�Ͽ������ռ��ѧ����ʽΪ________��
��2��1921�꣬_______������ĸ�������˽��Ƽ����ư���������������Ƽ����������ԭ�ϵ������ʡ�
A�������� B�������� C����°� D��������
������ʵ�顿ʳ���������Ƽ����Ҫԭ��֮һ������ƵõĴ����к��������Ȼ��ơ�Ϊ�˼��鴿����Ʒ�д��������ӣ���Ҫ�õ���ҩƷ��__________������ĸ����
A���Ȼ�����Һ B����������Һ C��ϡ���� D��ϡ����
������ʵ�顿��12.0g������Ʒ����ˮ�����������Ȼ�����Һ����ַ�Ӧ���ˡ�ϴ�ӡ����¸���õ�19.7g���������㴿����Ʒ��̼���Ƶ�����������__________��д��������̣��������һλС����
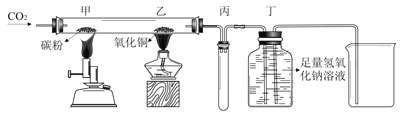
��ʵ����������ۡ���ͬѧ�������ͼʵ��װ�ã�ͨ������һ�������Ĵ�����Ʒ������ϡ���ᷴӦ�����������̼���������һ�����㴿����Ʒ��̼���Ƶ�����������

��1�����װ�õ�������ʱ����ʼ״̬�����ܺ�ˮ����Һ����ƽ����������ܣ�__________��֤����װ�õ����������á�
��2����ͬѧ��Ϊ��װ�ò�õĶ�����̼�������ƫС�������Dz����ϵ�ʧ������������______________________��
��3����ͬѧ��Ϊ��װ�ò�õĶ�����̼�������ƫ�����Dz����ϵ�ʧ������������______________________��
���𰸡� �մ� Na2CO3 + Ca(OH)2 2NaOH + CaCO3�� C BC 88.3% ��������Һ�����ˮ����Һ�棬��һ��ʱ����Һ��߶Ȳ�䣨�����������ɣ� ������̼������ˮ������ˮ��Ӧ ��õĶ�����̼������а���������ϡ���������������������ɣ�
�������������Ͽ�Ƭ����1��̼�����׳ƴ���մ� ���Դ������ʯ��Ϊԭ�Ͽ������ռ��ѧ����ʽΪNa2CO3 + Ca(OH)2��2NaOH + CaCO3������2��1921�꣬C����°����˽��Ƽ����ư���������������Ƽ����������ԭ�ϵ������ʡ�������ʵ�顿ʳ���������Ƽ����Ҫԭ��֮һ������ƵõĴ����к��������Ȼ��ơ�Ϊ�˼��鴿����Ʒ�д��������ӣ���Ҫ�õ���ҩƷ��B����������Һ��C��ϡ���ᡣ������ʵ�顿��12.0g������Ʒ����ˮ�����������Ȼ�����Һ����ַ�Ӧ���ˡ�ϴ�ӡ����¸���õ�19.7g������
����贿����Ʒ��̼���Ƶ�����Ϊxg
. BaCl2��Na2CO3 ��2NaCl��BaCO3��
106 197
X 19.7g
![]() ��
��![]() ,x��10.6g.
,x��10.6g.
������Ʒ��̼���Ƶ�����������![]() ��100����. 88.3% .
��100����. 88.3% .
��ʵ����������ۡ���1�����װ�õ�������ʱ����ʼ״̬�����ܺ�ˮ����Һ����ƽ����������ܣ���������Һ�����ˮ����Һ�棬��һ��ʱ����Һ��߶Ȳ�䣨�����������ɣ�, ֤����װ�õ����������á�
��2����ͬѧ��Ϊ��װ�ò�õĶ�����̼�������ƫС�������Dz����ϵ�ʧ�����������Ƕ�����̼������ˮ������ˮ��Ӧ�� ��3����ͬѧ��Ϊ��װ�ò�õĶ�����̼�������ƫ�����Dz����ϵ�ʧ�����������Dz�õĶ�����̼������а���������ϡ����������
�㾦�ñ�����Ҫ����̼�����ڲ�������ֽ����֯��ϴ�Ӽ��������ȷ����Ӧ�á�

 �Ƹ������������ϵ�д�
�Ƹ������������ϵ�д�����Ŀ����һ����ɫ���壬���ܺ����Ȼ��ơ��������ơ�̼���ơ������ƺ����ᱵ�е�һ�ֻ��֡�Ϊ̽������ɣ�ij����ѧϰС����Ʒ���������������ʵ�飺

��1������ʵ���У����˲����õ��IJ��������У��ձ�����������__________��
��2��С��ͬѧͨ������ʵ����֪����ɫ������Ʒ��һ������___________________��
һ������_________________��
��3��Ϊȷ����ɫ������Ʒ�п��ܴ��ڵ����ʣ�С��Գ���C����ʵ�顣
ʵ����� | ���� | ���� |
ȡ��������C���Թ��У��������_____________�� | �����ݲ���������ȫ����ʧ�� | ��ɫ������Ʒ��һ������__________�� |
С��ͬѧ��Ϊ��ɫ�����л������ʲ���ȷ�����Ƿ���ڣ��������ǣ��û�ѧ����ʽ��ʾ����_________________________________________________________________��
��Ҫȷ�ϣ�ֻ�轫����ʵ�鷽�������ӵ�һ���Լ���Ϊ______________���ɡ�
�������������������ʵĴ��ڶ�������������и���ʱ�����Ƚ���������ת������ѡ�����ǡ�����Լ��������ݲ�ͬ����ó����ۡ�
����Ŀ����ѧ��һ����ʵ��Ϊ�����Ŀ�ѧ��
��1�����Ʊ�����ʵ��̽������Ҫ����������±���
ʵ�� | ̽����������Ŀ����������������ʲô��ͬ | ̽�����ӵ��˶� | ̽������Ʒ��ʴ������ |
��� |
|
|
|
���� | ��ʵ����Ҫ���ƣ� �ټ���ƿ�����ͬ�� ��________��ͬ�� | ��ʵ��˵���� �ٷ����ڲ����˶��� ��Ӱ������˶����ʵ�������__________�� | ��ʵ��˵���� ����Ʒ��ʴ������֮һ����__________�Ӵ��� |
��2��ij��ѧѧϰС����ʵ������ϰ����һ������������������Һ��
������50g��������Ϊ6%���Ȼ�����Һ����Ҫ�Ȼ��Ƶ�����Ϊ__________g����������ƽ����������Ȼ���ʱ����ָ��ƫ�����̣�Ӧ__________������ĸ������ƽƽ�⡣
A�������Ȼ��� B�������Ȼ��� C�������ƶ����� D�������ƶ�����
�ڰ�50g��������Ϊ6%���Ȼ�����Һϡ�ͳ���������Ϊ4%���Ȼ�����Һ����Ҫˮ�����Ϊ__________mL��ˮ���ܶȿɽ��ƿ���1g/cm3��������Ͳ��ȡ�����ˮʱ�������Ӷ������õ����Ȼ�����Һ����������__________������ڡ�����С�ڡ����ڡ���4% ��