��Ŀ����
����Ŀ����ͭ��һ����Ҫ�Ľ���������ͭ��п�ĺϽ𣬿������������������������ճ���Ʒ��Ϊ�˲ⶨ��ͭ��Ʒ����ɣ�ȡ�����Ʒ�ֱ��ϡ���ᷴӦ����ʵ�����ݼ�¼���£�
��Ʒ | ��1�� | ��2�� | ��3�� | ��4�� | ��5�� |
ȡ��Ʒ������g�� | 40.0 | 40.0 | 40.0 | 40.0 | 40.0 |
ȡϡ����������g�� | 30.0 | 60.0 | 90.0 | 120.0 | 150.0 |
��������������g�� | 0.3 | 0.6 | 0.9 | 1.0 | 1.0 |
��Ҫ��ش��������⣺
��1������ʵ�����ݷ������ӵ��ݿ�ʼ�������Ѿ���Ӧ��ȫ�ˣ�
��2����ʽ����û�ͭ��Ʒ�н���п��������������Ҫ��д��������̣�
��3���ڸ���������ֽ�ϣ�����40.0g��Ʒ�м�ϡ�����������������������Ĺ�ϵ���ߣ� 
���𰸡�
��1��4
��2���⣺��40g��Ʒ�к�п������Ϊx
Zn+ | H2SO4 | = | ZnSO4+ | H2�� |
65 | 2 | |||
x | 1.0g |
![]()
���x=32.5g
���Ի�ͭ��Ʒ��п����������Ϊ ![]() ��100%=81.25%
��100%=81.25%
�𣺻�ͭ��Ʒ��п����������Ϊ81.25%
��3���⣺�������������Ϊ0�ǣ�û���������������Դ�0��ʼ����������IJ��ϼ��룬���������������ӣ������뵽120.0gʱ�������������������ӣ�Ϊһ��ֵ������40.0g��Ʒ�м�ϡ�����������������������Ĺ�ϵ����Ϊ��
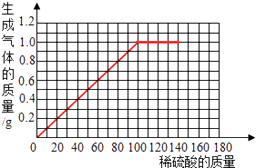
���������⣺��1����4�ݼ���120g������������1.0g����5�����ֶ����30g���ᣬ������������������û�䣬˵��п�Ѿ�����Ӧ���ˣ����Դӵ�4�ݿ�ʼ�������Ѿ���Ӧ���ˣ��ʴ�Ϊ����4�ݣ� ��1����4�ݼ���120g������������1.0g����5�����ֶ����30g���ᣬ������������������û�䣬˵��п�Ѿ�����Ӧ���ˣ���2�������������������������п������������п������������Ʒ���������ٷ�֮�ټ��ɣ���3���������������������Լ���Ҫ������������Ի���ϡ�����������������������Ĺ�ϵ���߽��н��

