��Ŀ����
����Ŀ��ˮ������֮Դ������ˮ��Դ�ǡ�������ɫ�������Ҫ����֮һ�� ��ش������й�ˮ������:
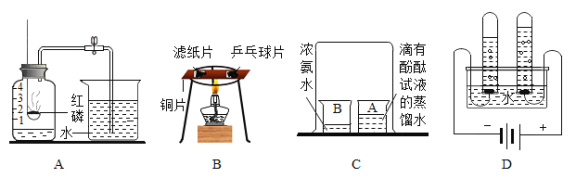
(1)������Ϊ���ڽ�Լ��ˮ����____________(�����);
A ϴ�������ʱ������ͷ
B ����Ϸ�ˮˢ��
C ������ˮ����Ϊ���ϳ�ˮ����
D ��ϴ��ˮ�����
(2)��ˮͨ��ɷ����ֽⷴӦ�����һ������ˮ������������������_________________(д��ѧʽ),����������������Ϊ5mLʱ�������������������Ϊ__________________mL��
����ȡ����ˮ���õ�������ClO2�Ļ�ѧ����ʽΪ: ![]() �� X�Ļ�ѧʽΪ___________________���������жϵ�������___________________________��
�� X�Ļ�ѧʽΪ___________________���������жϵ�������___________________________��
(3)����Ҫ���ij��ˮ�����ȣ���ѡ��____________________ (����ĸ���)��
A ��̪��Һ
B pH��ֽ
C ʯ����Һ
�������г���____________________�ķ���������ˮ��Ӳ�ȣ�ͬʱ��������ɱ����
���𰸡�A D O2 10 NaCl ��Ӧǰ��ԭ�ӵ��������Ŀ���� B ���
��������
��1��A��ϴ�������ʱ������ͷ���ܽ�Լ��ˮ��A��ȷ��
B������Ϸ�ˮˢ���������ˮ���˷ѡ������ڽ�Լ��ˮ��B����
C��������ˮ����Ϊ���ϳ�ˮ���£������ˮ���˷ѣ������ڽ�Լ��ˮ��C����
D����ϴ��ˮ�������һˮ���ã��ܽ�Լ��ˮ��D��ȷ��
�ʴ�Ϊ��AD��
��2����ˮͨ��ɷ����ֽⷴӦ�����һ������ˮ�������ڵ��ˮʱ�����������������������������������������������Ϊ2:1����������������������O2������������������Ϊ5mLʱ�������������������Ϊ10mL���ʴ�Ϊ��O2��10��
����ȡ����ˮ���õ�������ClO2�Ļ�ѧ����ʽΪ��Cl2+2NaClO2=2ClO2+2X���ɷ�Ӧ�Ļ�ѧ����ʽ��֪����Ӧǰ��2����ԭ�ӡ�4����ԭ�ӡ�4����ԭ�ӣ���Ӧ����2����ԭ�ӣ�4����ԭ�ӣ����2����ԭ�Ӻ�2����ԭ�ӡ���X�Ļ�ѧʽΪNaCl���������жϵ������Ǹ��������غ㶨�ɿ�֪����Ӧǰ��ԭ�ӵ����༰��Ŀ���䡣�ʴ�Ϊ��NaCl����Ӧǰ��ԭ�ӵ����༰��Ŀ���䣻
��3������Ҫ���ij��ˮ�����ȣ���ѡ��pH��ֽ����̪��Һ��ʯ����Һֻ�ܲⶨ��Һ������ԡ��ʴ�Ϊ��B��
�������г�����еķ���������ˮ��Ӳ�ȣ�ͬʱ��������ɱ�����ʴ�Ϊ����С�