��Ŀ����
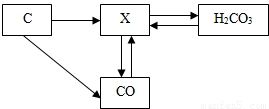
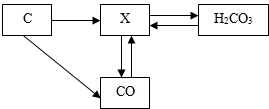
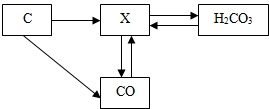
��ͼ��̼���仯�����ת����ϵͼ����ش��������⣺
��1�������ں���̼Ԫ�����ʵĻ�ѧʽ������������
��2��ת����ϵͼ����CO2��CaCO3�ķ�Ӧ��ѧ����ʽ��
�������������� ����
��3������þ��������������ȼ�գ�Ҳ�����ڶ�����̼��ȼ����������þ(MgO)��̼���ʣ�
þ�ڶ�����̼��ȼ�յĻ�ѧ����ʽΪ ��
�ɴ����ȼ�ա������ʲô�µ���ʶ�� ����һ��ɣ���
��4�������й�̼���仯�����˵����ȷ���� ��
A��������̼�ǵ����������Ҫ����
B����̼ȼ�ϲ���ȫȼ�ջ�����һ����̼��һ����̼�ж�
C��Һ̬������̼�������Ϊ����ʱ���ȣ������˿�ȼ����Ż��
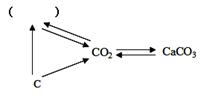 |
��1��CO (2��) ��2��CO2+Ca(OH)2 == CaCO3��+H2O (3��)
(3)2Mg+CO2 2MgO+C (3��)
ȼ�ղ�һ����Ҫ���� �����þ��ȼ�ղ�����CO2��� (2��)
(4)B(2��)

��ϰ��ϵ�д�
 ����С״Ԫ��������������ϵ�д�
����С״Ԫ��������������ϵ�д� ����������ϵ�д�
����������ϵ�д�
�����Ŀ
 ��̼ԭ�ӵ�����������Ϊ______��д��һ����֪����̼�ĵ��ʵ�����________��
��̼ԭ�ӵ�����������Ϊ______��д��һ����֪����̼�ĵ��ʵ�����________��