��Ŀ����
ij��ѧ��ȤС���ͬѧ��һƿ���õ���ʯ�ҷ�ĩ����ɽ���ʵ��̽��������һ��������ǵ�̽�����
[�������]��ƿ��ʯ�ҷ�ĩ�Ƿ��Ѿ�����������CaCO3��
[���в���]����һ����ʯ��ȫ�������CaCO3��
���������ʯ�Ҳ��ֱ����CaCO3��
����������ʯ��û�б��ʣ�
[���ʵ��]��С��ͬѧ�Բ���һ���������̽�����������������������±������ʵ�����ݣ�
| ʵ�鲽�� | ʵ������ | ʵ����� |
| ��ȡ����������ˮ�����裬���� ��ȡ������Һ���Թ��У������̪��Һ ��ȡ�����������Թ��У��������� | ��________�� ��________�� | ������� |
����ʯ�ұ���������________��Ե�ʣ��ڳ��л�ѧ������ѧ���������л���________������һ�����Ⱦ������������ʣ��������ʯ������������ʵ����Ӧ________���森
�۳�ȡ1.0g������ʯ����Ʒ��������ˮʹ֮����ܽ⣬������˺�����Һ�е����̪��Һ��ͬʱ����������������Ϊ10%����������Һ�պñ�Ϊ��ɫ������������Һ7.3g������Ʒ��Ca��OH��2������������д��������̣���
��Һ����ɫ �����ݲ��� ��Һ��� ������е�CO2������Ӧ Na0H �ܷ�
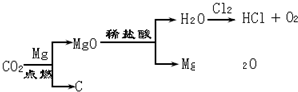
����������̽�����õ���ʯ�ҷ�ĩ����ɣ���Ҫ������ʯ����ˮ������еĶ�����̼��Ӧ����̼��ƶ����ʣ�
����ʯ�Ҳ��ֱ����CaCO3���û����;�����ʯ�Һ�̼��Ƶ����ʣ���������ˮ�����Һ�Լ��ԣ������������ᷴӦ�������壮
������ʯ����Ʒ��Ca��OH��2��������������Ҫ��������к͵Ļ�ѧ����ʽ���㣮
���[���ʵ��]����ʯ��ȫ�������CaCO3����ô��Һ�о�û����Ca��OH��2�����Ԣ�ȡ������Һ���Թ��У������̪��Һ
��Һ����ɫ����ȡ�����������Թ��У����������CaCO3�����ᷴӦ���ɶ�����̼���壬�������������ݲ�����
�ʴ�Ϊ������Һ����ɫ���������ݲ�����
�ٲ������ȷʱ����ҺΪCa��OH��2����Һ���Լ��ԣ���̪��죬�ʴ�Ϊ����Һ��죮
�ڱ�����Ҫ�ǽӴ������е�ˮ��Ͷ�����̼��Ӧ������ҩƷ�������������ʣ�
�ʴ�Ϊ��������е�CO2������Ӧ��Na0H���ܷ⣮
�۽⣺����Ʒ�к�Ca��OH��2������Ϊx
Ca��OH��2+2HCl=CaCl2+2H2O
74 73
x l0%��7.3g
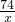 =
=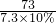
���x=0.74g
����Ʒ��Ca��OH��2����������ΪCa��OH��2%= ��100%=74%
��100%=74%
����Ʒ��Ca��OH��2��������Ϊ74%��
���������⿼����������׳��⣬�Ͷ�����̼��Ӧ�����������ʵ����ʣ�ѧ��Ӧ��Ϥ��صĻ�ѧ��Ӧ��
����������̽�����õ���ʯ�ҷ�ĩ����ɣ���Ҫ������ʯ����ˮ������еĶ�����̼��Ӧ����̼��ƶ����ʣ�
����ʯ�Ҳ��ֱ����CaCO3���û����;�����ʯ�Һ�̼��Ƶ����ʣ���������ˮ�����Һ�Լ��ԣ������������ᷴӦ�������壮
������ʯ����Ʒ��Ca��OH��2��������������Ҫ��������к͵Ļ�ѧ����ʽ���㣮
���[���ʵ��]����ʯ��ȫ�������CaCO3����ô��Һ�о�û����Ca��OH��2�����Ԣ�ȡ������Һ���Թ��У������̪��Һ
��Һ����ɫ����ȡ�����������Թ��У����������CaCO3�����ᷴӦ���ɶ�����̼���壬�������������ݲ�����
�ʴ�Ϊ������Һ����ɫ���������ݲ�����
�ٲ������ȷʱ����ҺΪCa��OH��2����Һ���Լ��ԣ���̪��죬�ʴ�Ϊ����Һ��죮
�ڱ�����Ҫ�ǽӴ������е�ˮ��Ͷ�����̼��Ӧ������ҩƷ�������������ʣ�
�ʴ�Ϊ��������е�CO2������Ӧ��Na0H���ܷ⣮
�۽⣺����Ʒ�к�Ca��OH��2������Ϊx
Ca��OH��2+2HCl=CaCl2+2H2O
74 73
x l0%��7.3g
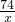 =
=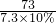
���x=0.74g
����Ʒ��Ca��OH��2����������ΪCa��OH��2%=
 ��100%=74%
��100%=74%����Ʒ��Ca��OH��2��������Ϊ74%��
���������⿼����������׳��⣬�Ͷ�����̼��Ӧ�����������ʵ����ʣ�ѧ��Ӧ��Ϥ��صĻ�ѧ��Ӧ��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
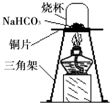 12��̼�����ƣ�NaHCO3���׳�С�մ�����ʳƷ��ҽҩ��ҵ��ij��ѧ��ȤС���ͬѧ��̼�����Ƶ����ʽ���̽����
12��̼�����ƣ�NaHCO3���׳�С�մ�����ʳƷ��ҽҩ��ҵ��ij��ѧ��ȤС���ͬѧ��̼�����Ƶ����ʽ���̽����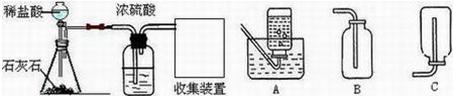
 ��2012?��̨��ij��ѧ��ȤС���ͬѧ����ͼ��ʾװ�ý���ʵ�飨װ�����������ã����ȹر�ֹˮ�У�������������������Һ������ƿ�У�������յ�������̼���ٴ�ֹˮ�У�
��2012?��̨��ij��ѧ��ȤС���ͬѧ����ͼ��ʾװ�ý���ʵ�飨װ�����������ã����ȹر�ֹˮ�У�������������������Һ������ƿ�У�������յ�������̼���ٴ�ֹˮ�У� ij��ѧ��ȤС���ͬѧ��ʵ����������������Ϊ8%������������Һ��������ⶨijϡ���������ʵ�����������
ij��ѧ��ȤС���ͬѧ��ʵ����������������Ϊ8%������������Һ��������ⶨijϡ���������ʵ�����������