��Ŀ����
ij����ÿ���ų���ˮ1000��3������ÿ������ˮ�к�������Cl2��35.5�ˣ������ᣨHCl��36.5�ˣ�ֱ���ŷŽ�����ˮ��Ⱦ���������������ƣ�Na2SO3����ȥCl2����Ӧ����ʽΪ��
Na2SO3+Cl2+H2O�TNa2SO4+2HCl������NaOH�к����ᣮ
���ʣ�1����1000��3��ˮ�к�HCl������Ϊ________�ˣ���ˮ��PH________7��ѡ�����������=������������
��2���ó�ÿ������������Ϊ9%��Na2SO3��Һ���ٿˣ�
��3��Ϊʹ��ˮ�����ԣ���NaOH������ٿˣ�
�⣺��1��3.65��l04 ��
��2������Ҫ9%Na2S03 ����Ϊx��������������y����
Na2S03+C12+H20�TNa2S04+2HCl
126 71 73
9%X 35500g Y
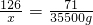
x=7.0��105g
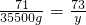
y=3.65��104g
��3���������������7.3 x 104��
����NaOH z��
HCl+NaOH�TNaCl+H20
36.5 40
7.3��l04g z
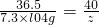
z=8.0��104g
����9%��Na2S03��Һ7.0��105�ˣ���NaOH����8.0��104�ˣ�
���������ݷ�ˮ���������������ɻ�ѧ����ʽ���HCl��������Na2SO3�������Na2SO3��Һ����������NaOH�����������
���������ݻ�ѧ����ʽ���㣬����Ҫд����ȷ�Ļ�ѧ����ʽ��Ȼ���ٸ�����֪��δ֪�����㣮
��2������Ҫ9%Na2S03 ����Ϊx��������������y����
Na2S03+C12+H20�TNa2S04+2HCl
126 71 73
9%X 35500g Y
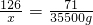
x=7.0��105g
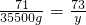
y=3.65��104g
��3���������������7.3 x 104��
����NaOH z��
HCl+NaOH�TNaCl+H20
36.5 40
7.3��l04g z
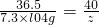
z=8.0��104g
����9%��Na2S03��Һ7.0��105�ˣ���NaOH����8.0��104�ˣ�
���������ݷ�ˮ���������������ɻ�ѧ����ʽ���HCl��������Na2SO3�������Na2SO3��Һ����������NaOH�����������
���������ݻ�ѧ����ʽ���㣬����Ҫд����ȷ�Ļ�ѧ����ʽ��Ȼ���ٸ�����֪��δ֪�����㣮

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
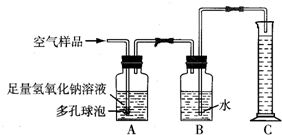 A��ij���������᳧���豸��ª�������¾ɣ��ó�ÿ���ŷŴ�����SO2�ķ����ͺ�H2SO4�����Է�ˮ�����ص����������;������ú̿��ȼ�ϣ�ֻҪ����������꣬�Ը�����ɼ����ƻ���
A��ij���������᳧���豸��ª�������¾ɣ��ó�ÿ���ŷŴ�����SO2�ķ����ͺ�H2SO4�����Է�ˮ�����ص����������;������ú̿��ȼ�ϣ�ֻҪ����������꣬�Ը�����ɼ����ƻ���