��Ŀ����
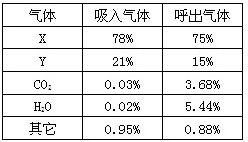 16��ij��ѧ��ȤС���ͬѧ���������ϵ�֪���˺����и������������������ұ���ʾ��
16��ij��ѧ��ȤС���ͬѧ���������ϵ�֪���˺����и������������������ұ���ʾ����1�������жϣ�����X��
������N2��
��Y��������O2��
����2����֤���˺����������к���ˮ��������ʵ�鷽����
���Ÿ���IJ�������
����3��X�����ں���������û�в��뻯ѧ��Ӧ�����ں������������������ȴ�����ˣ���ԭ���ǣ�
�������������������Ҫ�����ڶ�����̼��ˮ����������ĺ������Ӵ��������ļ��������
����������Ϊ������������ǿ��������ݿ������������������Ϳ����жϳ�X��Y��ʲô���ʣ���֤���˺����������к���ˮ������Ҫ����ˮ�����������������ˮ���������Ʒ����������ں�����������Ȼû�в��뻯ѧ��Ӧ���������������������Ѿ������˸ı䣬���µ����������Ҳ��仯��
����⣺����������������ǿ����������������ռ78%��X���ǵ����ˣ��������ռ21%��Y���������ˣ����ں����������к��д�����ˮ������ˮ����������IJ���Ƭ�ᱻ�����ˮ�飬��������Ƭ�Ϲ��������ˮ������û�й����IJ���Ƭ���ж��ռ��ɣ��ɱ������ݿ�֪�����������У�������̼��ˮ������������Ҫ�ȿ����м��ٵ�����࣬Ҳ����˵����������Ȼû�䣬�������������ȴ�����ˣ����Ե�����������������ˣ�
�ʴ�Ϊ����1������������
��2�����Ÿ���IJ���Ƭ����
��3���������������������Ҫ�����ڶ�����̼��ˮ����������ĺ������������������ļ����������
�ʴ�Ϊ����1������������
��2�����Ÿ���IJ���Ƭ����
��3���������������������Ҫ�����ڶ�����̼��ˮ����������ĺ������������������ļ����������
��������ȷ���������������ǿ��������ݿ����и�����ĺ������ж�X��Y���ɱ���������Ϣ������������������Ĺ�ϵ�ٽ��⣮

��ϰ��ϵ�д�
�����Ŀ
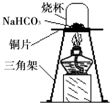 12��̼�����ƣ�NaHCO3���׳�С�մ�����ʳƷ��ҽҩ��ҵ��ij��ѧ��ȤС���ͬѧ��̼�����Ƶ����ʽ���̽����
12��̼�����ƣ�NaHCO3���׳�С�մ�����ʳƷ��ҽҩ��ҵ��ij��ѧ��ȤС���ͬѧ��̼�����Ƶ����ʽ���̽����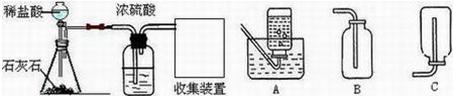
 ��2012?��̨��ij��ѧ��ȤС���ͬѧ����ͼ��ʾװ�ý���ʵ�飨װ�����������ã����ȹر�ֹˮ�У�������������������Һ������ƿ�У�������յ�������̼���ٴ�ֹˮ�У�
��2012?��̨��ij��ѧ��ȤС���ͬѧ����ͼ��ʾװ�ý���ʵ�飨װ�����������ã����ȹر�ֹˮ�У�������������������Һ������ƿ�У�������յ�������̼���ٴ�ֹˮ�У� ij��ѧ��ȤС���ͬѧ��ʵ����������������Ϊ8%������������Һ��������ⶨijϡ���������ʵ�����������
ij��ѧ��ȤС���ͬѧ��ʵ����������������Ϊ8%������������Һ��������ⶨijϡ���������ʵ�����������