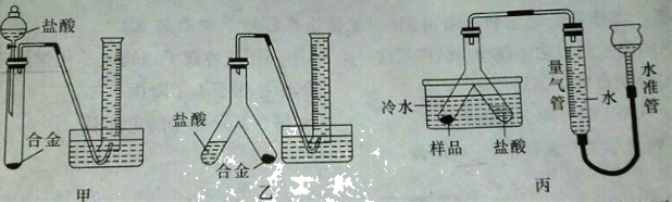
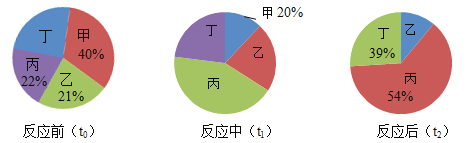
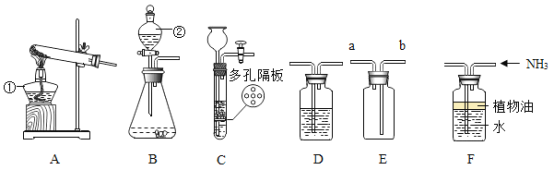
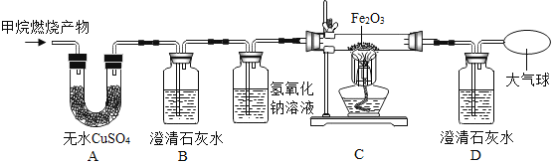
��Ŀ����
����Ŀ���±���Ca��OH��2��NaOH���ܽ�����ݣ���ش��������⣺
�¶�/�� | 0 | 20 | 40 | 60 | 80 | 100 |
�ܽ��/g | ||||||
Ca��OH��2 | 0.19 | 0.17 | 0.14 | 0.12 | 0.09 | 0.08 |
NaOH | 31 | 91 | 111 | 129 | 313 | 336 |
��1�������ϱ����ݣ�����Ca��OH��2��NaOH���ܽ�����ߣ�ͼ���ܱ�ʾNaOH�ܽ�����ߵ���_____���A����B������
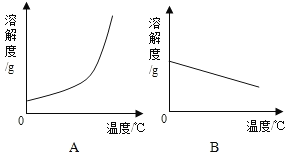
��2��Ҫ���һƿ�ӽ����͵�Ca��OH��2��Һ��ɱ�����Һ���ɲ�ȡ��ʩ��_____������ţ���
������ˮ���������¶ȡ��������¶ȡ�������ˮ����������������
��3������60��ʱ��Ca��OH��2��NaOH�������ʵı�����Һ����Ҫ�õ��ϴ�����NaOH���壬Ӧ��ȡ������������_____��
��4������20��ʱCa��OH��2�ı�����Һ������Һ���������м���һ����CaO��ָ�20�棬�õ�����Һ����Һ�����ʵ����������Ĺ�ϵΪ��_____�ң��������������������
���𰸡�A �٢ڢ� ��ȴ�ȱ�����Һ ��
��������
��1�����ݱ����ṩ�����ݿ��Կ������������Ƶ��ܽ�����¶ȵ����߶������ܱ�ʾNaOH�ܽ�����ߵ���A��
��2����һƿ�ӽ����͵�Ca��OH��2��Һ��ɱ�����Һ����������ˮ�������������ƹ��壬�����������Ƶ��ܽ�����¶ȵ����߶���С�����Բ��������¶ȵķ�������ѡ�٢ڢ���
��3���������Ƶ��ܽ�����¶ȵ����߶������������Ƶ��ܽ�����¶ȵ����߶���С���ʽ����¶���������������������������Ȼ��������Һ�У����Եõ��ϴ������������ƾ��壬�ʲ�ȡ��������������ȴ�ȱ�����Һ��
��4�����������Ƶı�����Һ�м��������ƣ�������������ˮ��Ӧ���ɵ����������ƣ����¶Ȼָ�ԭ�¶ȣ���Ȼ�Ǹ��¶��µı�����Һ�������������������䣬��Һ�����ʵ����������Ĺ�ϵΪ��=�ҡ�

 ��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�
��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�����Ŀ�������±���Ϣ�����˵����ȷ���ǣ�������
�¶�/�� | �ܽ��/g | |||
NaCl | KCl | NH4Cl | KNO3 | |
10 | 35.8 | 31.0 | 33.3 | 20.9 |
30 | 36.3 | 37.0 | 41.4 | 45.8 |
50 | 37.0 | 42.6 | 50.4 | 85.5 |
A.�������ʵ��ܽ�����¶�Ӱ����С����KNO3
B.30��ʱ��KCl������Һ��������������Ϊ37.0%
C.50�����µ�30����NH4Cl������Һ����������9.0g
D.30��ʱ����100gˮ�м���50gKNO3���壬����ܽ����Һ������Ϊ145.8g
����Ŀ���±��� KNO3��NaCl�ڲ�ͬ�¶��µIJ����ܽ�����ݡ�
�¶ȣ����� | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 |
KNO3 | 13.3 | 20.9 | 32.0 | 45.8 | 64.0 | 85.5 | 110.0 | 138.0 | 169.0 | 202.0 | 246.0 |
NaCl | 35.7 | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 | 37.3 | 37.8 | 38.4 | 39.0 | 39.8 |
��1���������������ܽ�ȵı仯���¶�Ӱ���С����_____��
��2����40��ʱ����40gKNO3�ܽ���50gˮ�У��γ���Һ��������_____g����������60�����γɵ���ҺΪ_____ ����
��3��50��ʱ������NaCl��Һ�����ʵ�����������w1���뱥��KNO3��Һ�����ʵ�����������w2���Ĵ�С��ϵ�ǣ�w1_____w2��������������=��������������
��4�����ݱ������ݣ��ҳ�50.0gˮ�ܹ��ܽ�55.0gKNO3���¶ȷ�Χ��_____��
��5������KNO3�л�������NaCl�����ᴿKNO3�����ʵ�鲽��Ϊ��������ˮ�ܽ������ȵõ��ȱ�����Һ��_____��������ϴ����������KNO3��
��6��������ˮ�е��ܽ��Ҳ�ж��ı仯���ɣ���ͼΪ��ͬ�¶��£�ij������ܽ�����¶ȵĹ�ϵ��ͼ��P1��P2��ʾ��ѹ����P1��P2�Ĺ�ϵ��_____��
A P1��P2 B P1=P2 C P1��P2 D ��ȷ��
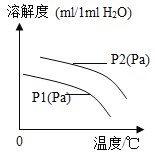
ͼ�л����Կ����¶ȶ������ܽ�ȵ�Ӱ��Ϊ_____��