��Ŀ����
����Ŀ���ⶨʯ��ʯ��̼��Ƶ�����������ȡ��ʯ��ʯ��Ʒ5g���� 60gϡ������Ĵμ��룬ʵ����������������±������ʲ�����ˮ��Ҳ����ϡ���ᷴӦ��������ʵ�����ݼ��㣺
ʵ����� | 1 | 2 | 3 | 4 |
����ϡ���������/g | 15 | 15 | 15 | 15 |
ʣ����������/g | 3.50 | 2.00 | 0.75 | 0.75 |
��1��ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ______________��
��2����ϡ��������������������______��
��3����Ӧ��������Һ����������������������_________��
���𰸡�85% 7.3% 7.6%
��������
�⣺��1���������ݿ�֪����������������ʱӦ��Ϊʣ������ʣ�����̼��Ƶ�����Ϊ5g-0.75g=4.25g��ʯ��ʯ��Ʒ��̼��Ƶ���������Ϊ![]() ��100%=85%��
��100%=85%��
��2�����ݵ�һ�μ���15gϡ���ᵼ�¹������5g-3.50g=1.50g����ԭ60gϡ������������������Ϊx��
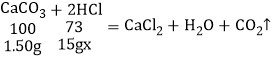
![]()
x=7.3%��
��3���裺��Ӧ�����������Ȼ��Ƶ�����Ϊm��ͬʱ���ɶ�����̼������Ϊn��
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 111 44
4.25g m n
![]() m=4.7175g��
m=4.7175g��
![]() n=1.87g��
n=1.87g��
��Ӧ��������Һ��������������������=![]() 100%=7.6%��
100%=7.6%��

��ϰ��ϵ�д�
�����Ŀ