��Ŀ����
ʵ���ҳ��õĸ��������ʯ�ҡ���CaO����NaOH�Ļ��������������ˮ������CO2��Ӧ�����ʣ�ijͬѧ��һƿ���õġ���ʯ�ҡ���������̽������1�������롿
�����û�б��ʣ�����ʯ�ҡ�ֻ����CaO������NaOH��
���������ȫ���ʣ�����ʯ�ҡ�ȫ�������CaCO3��Na2CO3��
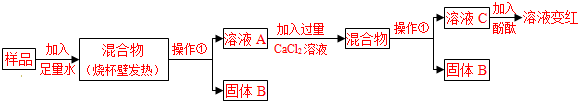
��2����ʵ�顿��ͼ��ʾ��

��3�����жϡ�
a����Ca��OH��2��CaCO3��Na2CO3Ͷ�뵽ˮ�в�����ȣ����ɲ����������жϣ������ �����������������
b�������ܷ�����ѧ��Ӧ�ķ���ʽΪ ���ɲ����ݢ������жϣ���Һ��һ������ ��д��ѧʽ�����ɴ��жϲ���� �������������������
c���ۺ�a��b�Ľ��ۣ��жϸ���Ʒ�������Ϊ ����д�������ʷ�Ӧ�Ļ�ѧ����ʽ �� ��
��4������չ��������ʵ��˵����ʵ�����С���ʯ�ҡ�Ӧ ���棻�������в����������� ��
���𰸡����������������ܹ��Ϳ����е�ˮ������ѧ��Ӧ�����������ƣ����������ƺ��������������Ͷ�����̼��Ӧ����̼��ƺ�̼���ƣ���˼�ʯ�Ҿ����ױ��ʣ��ɲ������е������ѵó���Ʒ��Ӧ�û����������ƻ�����������ƣ���Ϊ�����ƻ��������������ˮ����ȣ���˲���II�����������ݢݢ������ѵó���Һ��Ӧ����̼���ƣ��ʲ���I���������ݴ˷�����ɣ�
����⣺��3�����жϡ�a��Ca��OH��2��CaCO3��Na2CO3Ͷ�뵽ˮ�в�����ȣ���������зų�������˵�������к��������ƻ��������ƣ����Բ��������
b���������м���ϡ����������壬����֪���������к���̼��ƣ��䷽��ʽΪ��CaCO3+2HCl=CaCl2+CO2��+H2O������Һ�м���ϡ������������ƺ����������ͳ����������ж�����Һ�к���̼���ƣ���˵���ڹ�������̼��ƺ�̼���ƣ����Բ��������
c�����ʵ�飬����֪���ڹ����к���̼��ƣ�̼���ƺ������ƻ����������ƣ��ʿ����жϸ�����Dz��ֱ��ʣ��������Ƶ���֪���ܷ����ı��ʷ�ӦΪ����������ˮ�����������������̼�����������������̼�ȣ�
����չ�������ܹ��Ϳ����еijɷַ�Ӧ���������ʵ����ʣ������ܷⱣ�棬�ڹ���ʱ�����������þ�����������ֹ��Һ�⽦��
�ʴ�Ϊ����3�����жϡ�a����������
b��CaCO3+2HCl=CaCl2+CO2��+H2O��Na2CO3����������
c��������Dz��ֱ��ʣ�CaO+H2O=Ca��OH��2��2NaOH+CO2�TNa2CO3+H2O��
����չ���ܷ⣻��������ֹ��Һ�⽦��
��������������̼���ơ�̼��Ƶ�̼���κ��������ơ������ƵĻ�ѧ���ʣ����ܸ���������ʵ������Լ����ʵ������֤�����Ƿ�����ǽ���Ĺؼ���
����⣺��3�����жϡ�a��Ca��OH��2��CaCO3��Na2CO3Ͷ�뵽ˮ�в�����ȣ���������зų�������˵�������к��������ƻ��������ƣ����Բ��������
b���������м���ϡ����������壬����֪���������к���̼��ƣ��䷽��ʽΪ��CaCO3+2HCl=CaCl2+CO2��+H2O������Һ�м���ϡ������������ƺ����������ͳ����������ж�����Һ�к���̼���ƣ���˵���ڹ�������̼��ƺ�̼���ƣ����Բ��������
c�����ʵ�飬����֪���ڹ����к���̼��ƣ�̼���ƺ������ƻ����������ƣ��ʿ����жϸ�����Dz��ֱ��ʣ��������Ƶ���֪���ܷ����ı��ʷ�ӦΪ����������ˮ�����������������̼�����������������̼�ȣ�
����չ�������ܹ��Ϳ����еijɷַ�Ӧ���������ʵ����ʣ������ܷⱣ�棬�ڹ���ʱ�����������þ�����������ֹ��Һ�⽦��
�ʴ�Ϊ����3�����жϡ�a����������
b��CaCO3+2HCl=CaCl2+CO2��+H2O��Na2CO3����������
c��������Dz��ֱ��ʣ�CaO+H2O=Ca��OH��2��2NaOH+CO2�TNa2CO3+H2O��
����չ���ܷ⣻��������ֹ��Һ�⽦��
��������������̼���ơ�̼��Ƶ�̼���κ��������ơ������ƵĻ�ѧ���ʣ����ܸ���������ʵ������Լ����ʵ������֤�����Ƿ�����ǽ���Ĺؼ���

��ϰ��ϵ�д�
 ���ѵ����Ԫ��ĩ���100��ϵ�д�
���ѵ����Ԫ��ĩ���100��ϵ�д� ��˼άС�ھ�100����ҵ��ϵ�д�
��˼άС�ھ�100����ҵ��ϵ�д� ��ʦָ��һ��ͨϵ�д�
��ʦָ��һ��ͨϵ�д�
�����Ŀ




 ��ʯ����ʵ���ҳ��õĸ������ͬѧ��Ϊȷ��һƿ���õġ���ʯ�ҡ��������Ѳ��ֱ��ʻ�ȫ�����ʣ���Ʒ�ijɷ֣���������̽����
��ʯ����ʵ���ҳ��õĸ������ͬѧ��Ϊȷ��һƿ���õġ���ʯ�ҡ��������Ѳ��ֱ��ʻ�ȫ�����ʣ���Ʒ�ijɷ֣���������̽����