��Ŀ����
������ͼ��ʵ��ش����⣺

(1)С��ͬѧ����ͼʵ����ּ���ƿ�ײ�ը���ˣ�˵��ͼ�еĴ�����_____��
(2)��ͼ��ȴ�ȵ��������Һ���Թܵײ��о����������ɴ˵ó������¶Ƚ��ͣ�����ص��ܽ��____��ԭ���ȵĶ��������Һ______(��ǡ����ǡ���һ���ǡ�)������Һ��
(3)��ͼ��̽��ȼ��������ʵ�飬��Һ©���Ļ���ǰ�����ײ�ȼ�գ��������ȼ�գ��Ƚϴ�ǰ�������֤��������ȼ����Ҫ____���ձ���ˮ��������___����ƿ�ڷ�����Ӧʱ�Ļ�ѧ����ʽΪ___��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����ʵ�鷽���ܴﵽʵ��Ŀ�ĵ���( )
ѡ�� | ʵ��Ŀ�� | ʵ�鷽�� |
A | ��ȥCO2�е�HCl���� | ͨ��NaOH��Һϴ�� |
B | ֤��������̼����ˮ��Ӧ | ��������̼ͨ����з�̪��Һ��ˮ�� |
C | �����������ռ��� | �������ǵ�ľ�����뼯��ƿ�� |
D | ֤�������к���̼Ԫ�� | ���ڱ�պ�г���ʯ��ˮ���ձ�������������Ϸ� |
A.A B.B C.C D.D
������;�㷺����ṹ�����ʵ��ǻ�ѧ����Ҫ�о����ݡ�
��1����ͼ������ԭ�ӽṹʾ��ͼ������˵������ȷ����_____��
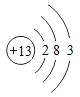
A ��ԭ�ӵ�������Ϊ13 B �ڻ���������ͨ����+3��
C ���ǵؿ��к�������Ԫ�� D �������������������������õĵ�����
��2��ij��ѧС����һ����AgNO3��Cu(NO3)2�����Һ��������ͼʵ�飬������ҺA����B�ijɷֽ����˷�����ʵ��̽����
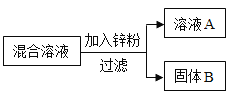
��������⣩��ҺA�е����ʿ�������Щ��
���������룩��ֻ��Zn(NO3)2��Zn(NO3)2��AgNO3��Zn(NO3)2��Cu(NO3)2��Zn(NO3)2��AgNO3��Cu(NO3)2
���������ۣ��������IJ�����_____�����ţ�����������_____��
��ʵ��̽����������ٳ�����ͨ������ʵ���ȷ������B�ijɷ֣��뽫�±���д������
ʵ�鲽�� | ���� | �йط�Ӧ�Ļ�ѧ����ʽ |
ȡ��������B���μ�_____ | �����ݲ��� | _____ |
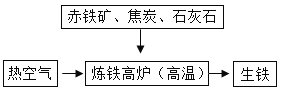
��3����ͼ�ǹ�ҵ����ʾ��ͼ�����У���̿��������ȼ���ṩ������_____���������ɵĻ�ѧ����ʽΪ_____��
��4��ij�������÷���м���������Ӧ����ȡ�������������з�����49t��H2SO4����������Ϊ10%��������������м��Ӧ������������������������_____


 B.
B. C.
C. D.
D.

