��Ŀ����
ijѧϰС��ȡ25gʯ��ʯ��Ʒ�����ձ��У�����ϡ���������ٲ�������Ϊֹ��ʯ��ʯ�е����ʲ�����ˮ��������Ԫ�أ�Ҳ����ϡ���ᷴӦ��������ϡ�����������ų����������Ĺ�ϵ��ͼ��
��1 ����Ʒ�е�̼�����ȫ��Ӧʱ����ϡ���������Ϊ______g��
��2������ϡ��������Ʒ�е�̼���ǡ����ȫ��Ӧ������ȫ���ݳ�������ʱ�ձ�����Һ�������������ʣ���������Ϊ______g��
��3����ʯ��ʯ��Ʒ�и�Ԫ�ص������Ƕ��ٿˣ����淶д��������̣�
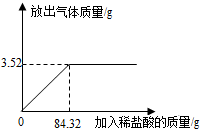
��1 ����Ʒ�е�̼�����ȫ��Ӧʱ����ϡ���������Ϊ______g��
��2������ϡ��������Ʒ�е�̼���ǡ����ȫ��Ӧ������ȫ���ݳ�������ʱ�ձ�����Һ�������������ʣ���������Ϊ______g��
��3����ʯ��ʯ��Ʒ�и�Ԫ�ص������Ƕ��ٿˣ����淶д��������̣�

����1��ͼ���ת�۵��Ƕ���ǡ�÷�Ӧ�ĵ㣬�۲�ͼ���֪��ʱ��ȥϡ���������Ϊ84.32g��
�ʴ�Ϊ��84.32g��
��ͼ���֪������Ʒ��������ᷴӦ���ɶ�����̼3.52g��
��̼��Ƶ�����Ϊx
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
x3.52g
=
��ã�x=8g
��2���ձ�����Һ��������Ϊ��8g+84.32g-3.52g=88.8g
�ʴ�Ϊ��88.8g��
��3����ʯ��ʯ��Ʒ�и�Ԫ�ص�����Ϊ��8g��
��100%=3.2g
�𣺸�ʯ��ʯ��Ʒ�и�Ԫ�ص�����Ϊ3.2g��
�ʴ�Ϊ��84.32g��
��ͼ���֪������Ʒ��������ᷴӦ���ɶ�����̼3.52g��
��̼��Ƶ�����Ϊx
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
x3.52g
| 100 |
| 44 |
| x |
| 3.52g |
��ã�x=8g
��2���ձ�����Һ��������Ϊ��8g+84.32g-3.52g=88.8g
�ʴ�Ϊ��88.8g��
��3����ʯ��ʯ��Ʒ�и�Ԫ�ص�����Ϊ��8g��
| 40 |
| 40+12+16��3 |
�𣺸�ʯ��ʯ��Ʒ�и�Ԫ�ص�����Ϊ3.2g��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ