��Ŀ����
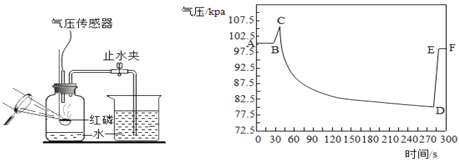
����Ŀ��ij��ѧ��ȤС�����������̼������̽�������˶������ݴ���������ʵʱ���CO2���������ʱ��仯�����ߡ��ռ�һ����ƿCO2������������ʵ�飺�ٳ��ڷ��ã���˫����������ƿ����ϣ���˫�ֽ�������תƿ�ڡ�����ʵ���в��CO2�����������ʱ��仯����������Ϊ��ͼ�е�MN�Ρ�NP�κ�PR�Ρ�����˵������ȷ���ǣ���
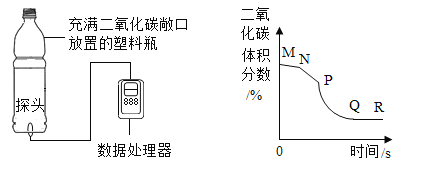
A.������M��N�ı仯���̣�˵��CO2�����Dz����˶���
B.������M��N��N��P��Ƚϣ�˵���¶�Խ�߷����˶��ٶ�Խ��
C.������N��P��P��Q��Ƚϣ�˵��CO2�ܶȱȿ����ܶȴ�
D.������P��O��Q��R��Ƚϣ�˵��Q���CO2���ӵ��˶��ٶȼ���
���𰸡�D
��������
A��������M��N�ı仯���̣�������̼��������������½���˵��CO2�����Dz����˶��ģ���ѡ����ȷ�������������⣻
B��������M��N��N��P��Ƚϣ�N��P�½��ٶȽϿ죬˫����������ƿ�������ƿ���¶����ߣ�˵���¶�Խ�߷����˶��ٶ�Խ�죻��ѡ����ȷ���������������⣻
C��������N��P��P��Q��Ƚϣ�P��Q�½��Ͽ죬˫�ֽ�������תƿ�ڣ�������̼��ƿ���˶���˵��CO2�ܶȱȿ����ܶȴ�ѡ����ȷ�������������⣻
D��������P��Q��Q��R��Ƚϣ�������̼���������С������Ϊ̽ͷ���ڵ�������ƿ���϶ˣ�������̼���ܶȴ�������ƿ���¶˽϶࣬��̽ͷ�Ӵ��Ļ����٣�����˵��Q���CO2���ӵ��˶��ٶȼ�������ѡ�����
��ѡ��D��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ��I.�ڻ�ѧ��ѧϰ�У�ͬѧ��֪��������ϩ����ֻ��C��H����Ԫ�أ����÷��շ��������������ϻ���ɴ�����Ⱦ����ѧ��ȤС��Ծ���ϩ�����ڿ�����ȼ�����ɵ�̼����������ɽ���̽����
��������룩A.ֻ��CO2 B.ֻ��CO C_____��
���������ϣ���CO����û����������Һ���Ȼ��ٻ�ɫ��ֽ��������������CO2������ֽ����ɫ��
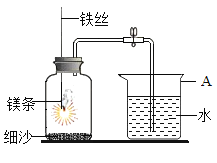
����Ʒ�������ѧ��ȤС������ʦ��ָ���£������ͼ��ʾʵ�飬ͨ���۲�װ��B��װ��C��ʵ��������֤���롣
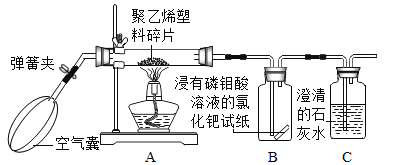
��ʵ��̽������������������ʵ�鱨�档
ʵ�鲽�� | ʵ������ | ʵ����ۼ���ѧ����ʽ |
��ȼ�ƾ��Ƽ��ȣ��漴���ɼУ�ͨ�����Ŀ����� | װ��B�е�������_____�� װ��C�е�������_____�� | ����ϩ�����ڿ�����ȼ�գ�����̼���������У�����CO2��Ҳ��CO��װ��C�з�Ӧ�Ļ�ѧ����ʽΪ_____�� |
�����ۣ�
��1������ʵ����ۣ��ӻ����Ƕȿ��ǣ�ʵ����Ӧ��β�����д���������������_____��
��2������ϩ�����ڿ�����ȼ�գ��������г���CO2��CO�⣬��Ӧ�е�������_____��
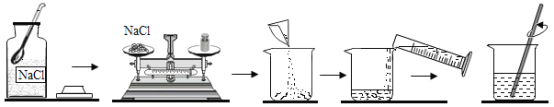
����Ŀ��̼����(SrCO3)�����������ɫ���桢ӫ�ⲣ���ȡ�С��ͬѧΪ�ⶨijSrCO3��NaCl����������SrCO3�������������������ձ��н������ʵ�飬ʵ������ϡ��������ʵ�����������ͬ�������������±���ʾ��
�ձ���� | �� | �� | �� |
ϡ��������/g | 100 | 200 | 300 |
������������/g | 100 | 100 | 100 |
��Ӧ���ձ������ʵ�����/g | 195.6 | 291.2 | 391.2 |
����
(1)�ձ����в���CO2������Ϊ____g��
(2)ԭ����������SrCO3����������____(��������ȷ��0.1%)��
(��ʾ��SrCO3+2HCl===SrCl2+CO2��+H2O)